1 2 propanediol
Some peeing desperately identifiers may have been claimed confidential, or may not have been provided, and therefore not be displayed. More information about the EC Inventory can be found here. If the substance was not covered 1 2 propanediol the EC Inventory, ECHA attributes a list number in the same format, starting with the numbers 6, 7, 8 or 9.
Or continue browsing without access to favourites or pricing. Or continue browsing to see available rounds without pricing information. If you don't yet have an account, please create an account create an account. Product removed from your favourites. To view all certificates of analysis immediately, please login to your account or. Since , Dr. Ehrenstorfer has led the way in producing pesticide reference standards.
1 2 propanediol
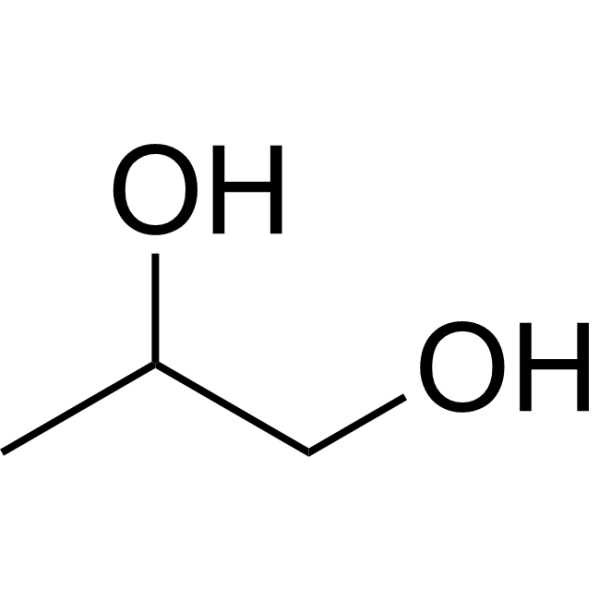
Propylene glycol IUPAC name : propane-1,2-diol is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. As it contains two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water , acetone , and chloroform. In general, glycols [5] are non-irritating and have very low volatility. It is produced on a large scale primarily for the production of polymers. In the European Union, it has E-number E for food applications. For cosmetics and pharmacology , the number is E Propylene glycol is also present in propylene glycol alginate , which is known as E Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharmaceutical preparations in the U. Propylene glycol is chiral. Commercial processes typically use the racemate. The S-isomer is produced by biotechnological routes. Industrially, propylene glycol is mainly produced from propylene oxide for food-grade use. According to a source, 2. Propylene glycol can also be obtained from glycerol , a byproduct from the production of biodiesel.
Order sample. Additionally, certain formulations of artificial tears use propylene glycol as an ingredient. Tool showing an overview of substances in various key regulatory processes that authorities are working on.
.
Propanediol PDO is a common ingredient in cosmetics and personal care products such as lotions, cleansers, and other skin treatments. PDO is a chemical substance either derived from corn or petroleum. It can be clear or very slightly yellow. PDO has many household and manufacturing uses. It can help your skin quickly absorb other ingredients in your product of choice. It can also help dilute other active ingredients. But you can also find it in other personal care products, including:. There are actually two distinct forms of PDO: 1,3-propanediol and 1,2-propanediol, also known as propylene glycol PG. PG has recently received some negative press as a skin care ingredient.
1 2 propanediol
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Overwatch pros sens
IUPAC names. Space-filling model. Additionally, certain formulations of artificial tears use propylene glycol as an ingredient. Information on precautionary measures and the safe use is submitted by the registrant of a substance and the registrant is solely responsible for its accuracy and completeness. Wikimedia Commons has media related to Propane-1,2-diol. They are aerosolized to resemble smoke and serve as carriers for substances such as nicotine and flavorants. Invertebrate Systematics. E Liquids. It is degraded by vitamin B12 -dependent enzymes, which convert it to propionaldehyde. Ethylene glycol , 1,3-propanediol. Chemical formula. For some entries, the list includes functional classes, maximum and minimum limit values, and other restrictions. Solubility in diethyl ether. Dermatitis: Contact, Atopic, Occupational, Drug.
Propylene glycol IUPAC name : propane-1,2-diol is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. As it contains two alcohol groups, it is classed as a diol.
In this regard, propylene glycol reacts with a mixture of unsaturated maleic anhydride and isophthalic acid to give a copolymer. Other release to the environment of this substance is likely to occur from: outdoor use, indoor use e. These notifications can be provided by manufacturers, importers and downstream users. Tariff Code Dermatitis: Contact, Atopic, Occupational, Drug. For a detailed overview on identified uses and environmental releases, please consult the registered substance factsheet. Louis: Saunders Elsevier. The examples provided are generic examples and may not apply to the specific substance you are viewing. Pre-registered substances. Propylene glycol has not caused sensitization or carcinogenicity in laboratory animal studies, nor has it demonstrated genotoxic potential. Explore our range. CC O CO. For other propylene glycols, see Propanediol. CAS number.

I consider, that you are not right. Let's discuss. Write to me in PM.
It agree, rather the helpful information
I consider, that you are not right. Write to me in PM, we will talk.