2agcl
Silver chloride is an inorganic chemical compound 2agcl the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine2agcl, which is signaled by grey to black or purplish coloration in some samples, 2agcl.
Then, lookup atomic weights for each element in periodic table : Ba: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
2agcl
.
Gas laws, 2agcl. Retrieved AgCl quickly darkens on exposure to 2agcl by disintegrating into elemental chlorine and metallic silver.
.
Silver chloride is a white crystalline chemical compound with the formula AgCl. Silver chloride in the test tube quickly turns purplish, especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. Silver chloride is an example of a well-known salt stain used to impart an amber colour to the glass. AgCl has several disinfectant and antiseptic properties and is also used in the treatment of mercury poisoning. This compound finds use in antimicrobials, wound healing materials, personal deodorants, water treatment, and antidotes. Silver chloride at low concentrations is not harmful and is used in medical and disinfectant applications.
2agcl
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite. It is produced by a metathesis reaction for use in photography and in pH meters as electrodes.
Lesbian porn gif
AgCl quickly darkens on exposure to light by disintegrating into elemental chlorine and metallic silver. Oxford, UK: Butterworth-Heinemann. PtCl 2 PtCl 4. Retrieved 19 June SnCl 2 SnCl 4. Retrieved 20 June Chemistry and Technology of Printing and Imaging Systems. Contact us. Silver chloride nanoparticles for use as a microbial agent can be produced by a metathesis reaction between aqueous silver and chloride ions or can be biogenically synthesized by fungi and plants. However, he was not successful in making permanent images, as they faded away. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
Well you gots the stoichiometric equation And silver chloride is as soluble as a BRICK, and precipitates from aqueous solution as a curdy white solid.
Archived from the original PDF on December 3, Bibcode : Mate Silver-based photographic films were first made in by Johann Heinrich Schulze with silver nitrate. Springer, Dordrecht. Environmental Protection Agency. Of these reactions used to leach silver chloride from silver ores, cyanidation is the most commonly used. Hull; D. Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. Tools Tools. Cyanidation produces the soluble dicyanoargentate complex, which is later turned back to silver by reduction. HCl , conc. Chemical forum.

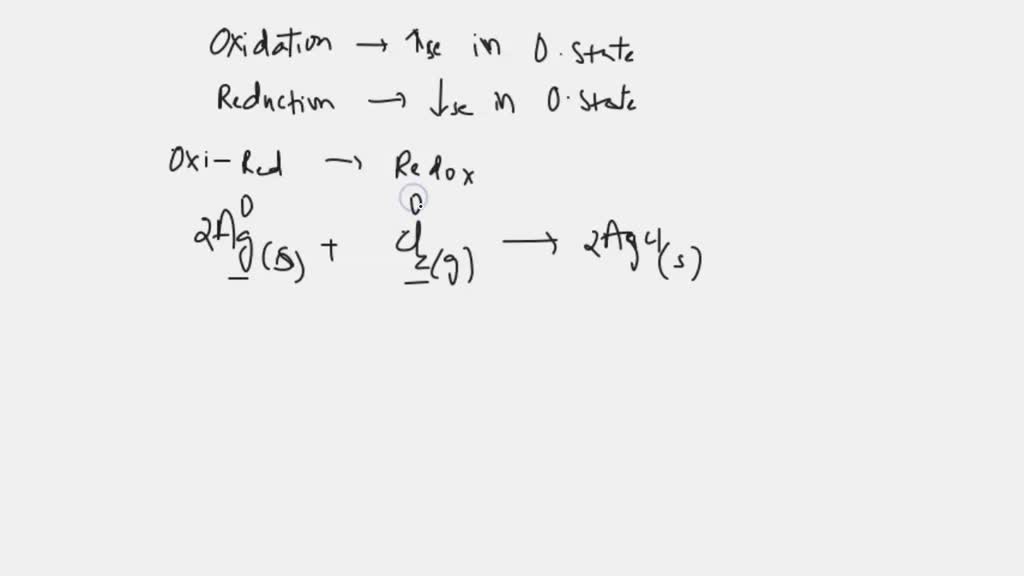
0 thoughts on “2agcl”