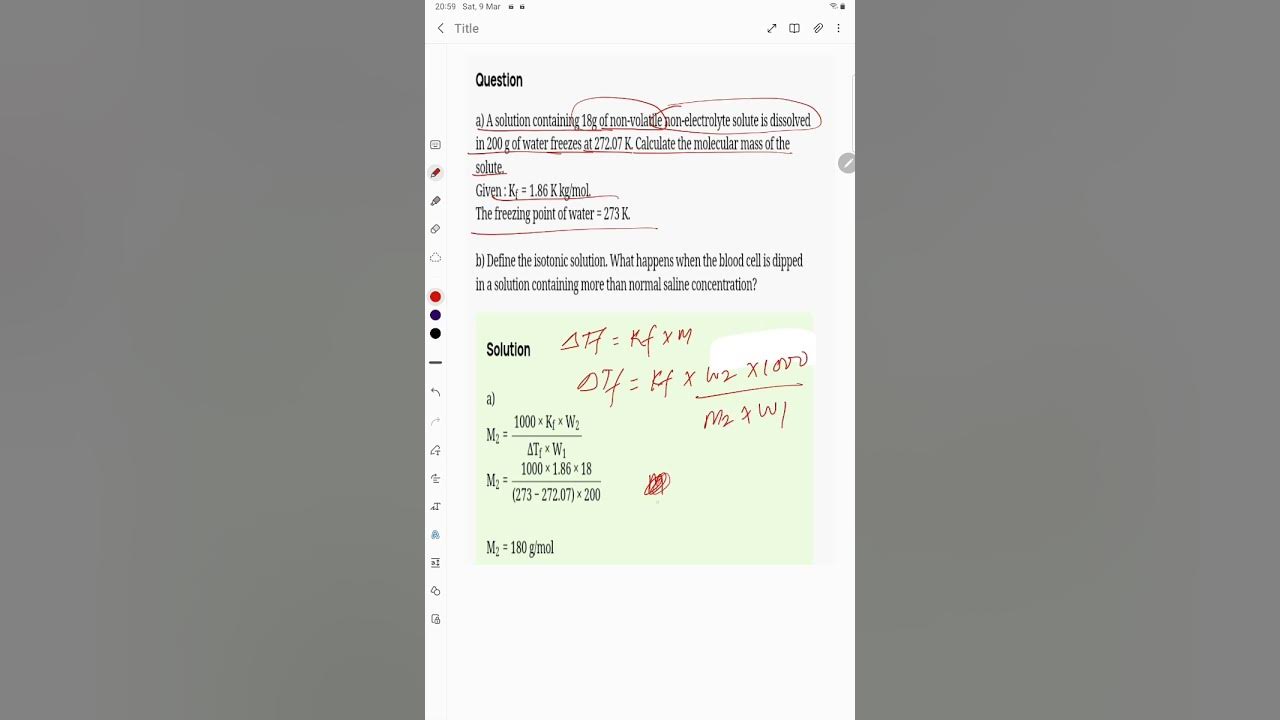
A solution containing 18g of non volatile
Posted by Maphisabet Jeengaphs 3 years, 7 months ago. Gaurav Seth 3 years, 7 months ago. Q : A solution containing 18g of non-volatile non-electrolyte solute is dissolved in g of water freezes at
Write equations for the steps in S N 1 mechanism of the conversion of tert. Describe the three steps involved in the leaching of bauxite to get pure alumina equations not expected. An element having atomic mass A solution of 1. A solution containing 6. Mass of solute is. The freezing point of a solution containing 18 g of a non-volatile solute dissolved in g of water decreases by 0.
A solution containing 18g of non volatile
A solution containing 18 g of anon-volitile solute in g of waer freezes at Calculate the molecular mass of the solute. State Raoult's law. Why is the vapour pressure of a solvent lowered by the addition of nonvolatile solute to it? A solution containing 18 g of non-volatile solute in g of water freezes at A solution containing 18 g of a non - electrolytic solute in g of H 2 O freezes at Find the molecular mass of the solute. A solution of 1. Calculate the molecular mass of the solute, K f for water is 1. A solution containing
Find the molecular mass of the solute. Remember the goal of this website is to share knowledge and learn from each other. K f for water is 1.
.
Volatility is the tendency of a substance to vaporize. Volatile substances: Volatile substances have the capability to go into the vapor phase. This may happen during heating or without heating. Volatility and the vapor pressure of a substance are related. If the volatility is high, the vapor pressure is also high. If the volatility is low, then the vapor pressure is low. Normally liquids are volatile. They tend to go into the vapor phase rapidly. For example, acetone, hexane, chloroform are volatile liquids, which evaporates rapidly.
A solution containing 18g of non volatile
This page deals with Raoult's Law and how it applies to solutions in which the solute is non-volatile - for example, a solution of salt in water. A non-volatile solute the salt, for example hasn't got any tendency to form a vapour at the temperature of the solution. It goes on to explain how the resulting lowering of vapour pressure affects the boiling point and freezing point of the solution. Important: If you haven't already read the page about saturated vapour pressure , you should follow this link before you go on. Use the BACK button on your browser to return to this page when you are ready. There are several ways of stating Raoult's Law, and you tend to use slightly different versions depending on the situation you are talking about. You can use the simplified definition in the box below in the case of a single volatile liquid the solvent and a non-volatile solute. In this equation, P o is the vapour pressure of the pure solvent at a particular temperature. That is exactly what it says it is - the fraction of the total number of moles present which is solvent.
Local time in south carolina
Close Answer. A solution containing 18 g of anon-volitile solute in g of waer fr Find the molecular mass of the solute. Calculate the molecular mass of the solute. A solution of 7 g of a non-volatile solute in g of water boils at A solution containing 18 g of non-volatile solute in g of water fre Find the molecular mass of solute. A solution containing 18 g of a non - electrolytic solute in g of H 2 O freezes at Describe the three steps involved in the leaching of bauxite to get pure alumina equations not expected. Posted by Richa Reji 2 weeks ago. A solution containing 18g of non - volatile non - electrolyte solute is dissolved in g of water freezes at What is reducing sugar and non reducing sugar. Posted by Nikita Bhatt 3 weeks, 4 days ago. The freezing point of a solution containing 18 g of a non-volatile sol
Submitted by Lisa W.
A solution containing 6. Calculate the freezing point of a solution that contains 30 g urea in g water. A solution of 1. Define isotonic solution. A solution containing 18 g of non-volatile solute in g of water fre View Solution. Write any two differences between physisorption and chemisorption. This content has been hidden. Find the molecular mass of solute. Recommended Questions. A solution containing 18 g of a non volatile solute in g of H2O freezes at Write equations for the steps in S N 1 mechanism of the conversion of tert. Name the crystal defect which lowers the density in an ionic crystal. If K f for water is 1.

I regret, that I can help nothing. I hope, you will find the correct decision. Do not despair.
Quite good question