Agcl molar mass
Laboratory Notes. The molecular weight of Silver chloride [AgCl] is Silver chloride [AgCl] is an inorganic compound of two elements: Silver and Chlorine.
Molar mass of AgCl Silver chloride is Then, lookup atomic weights for each element in periodic table : Ag: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Agcl molar mass
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite. It is produced by a metathesis reaction for use in photography and in pH meters as electrodes. Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis : combining an aqueous solution of silver nitrate which is soluble with a soluble chloride salt, such as sodium chloride which is used industrially as a method of producing AgCl , or cobalt II chloride. The silver chloride that forms will precipitate immediately. It can also be produced by the reaction of silver metal and aqua regia ; however, the insolubility of silver chloride decelerates the reaction. Silver chloride is also a by-product of the Miller process , where silver metal is reacted with chlorine gas at elevated temperatures. Silver chloride has been known since ancient times.
Chemistry tools. Oxygen O has an atomic mass of about
.
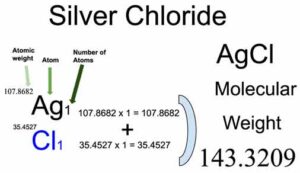
Silver chloride is described as a white crystalline chemical compound having the formula AgCl. Silver chloride, present in the test tube, turns purplish quickly, especially in the case of a sunny laboratory due to the silver chloride being split up into both chlorine and silver. Silver chloride can be prepared when the sodium chloride compound is added to the silver nitrate solution; there occurs a white precipitate of silver chloride. Silver chloride is also an example of a well-known salt stain, which is used to impart an amber colour to the glass. Chloro silver is the other name of silver chloride. Let us look at the properties of silver chloride as follows. Silver Chloride. Molar Mass or Molecular Weight.
Agcl molar mass
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Garou wallpaper
Studies in Conservation. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Due to its conspicuousness, it is easily used in titration, which gives the typical case of argentometry. PMID SbCl 3 SbCl 5. Your email address will not be published. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. The molar mass of carbon dioxide is In one of the most famous reactions in chemistry, the addition of colorless aqueous silver nitrate to an equally colorless solution of sodium chloride produces an opaque white precipitate of AgCl: [14]. Crystal structure. Retrieved 28 July Weights of atoms and isotopes are from NIST article. Araujo SnCl 2 SnCl 4.
Uses the formula of a reactant to determine molar mass.
Your email address will not be published. HCl , conc. As an example of the latter, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in seawater environments. PtCl 2 PtCl 4. EuCl 2 EuCl 3. One mole contains exactly 6. The history of the discovery of photography. Bibcode : Mate Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Silver bromide slightly yellowish white and silver iodide bright yellow are also significantly more photosensitive than is AgCl.

Now all became clear, many thanks for the information. You have very much helped me.
I consider, that you are not right. Let's discuss. Write to me in PM.
I can not take part now in discussion - there is no free time. But I will soon necessarily write that I think.