Are ionic bonds stronger than covalent bonds
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact. But do atoms ever actually touch one another? Most physicists would say no, because the negatively charged electrons in their valence shells repel one another. No force within the human body—or anywhere in the natural world—is strong enough to overcome this electrical repulsion, are ionic bonds stronger than covalent bonds.
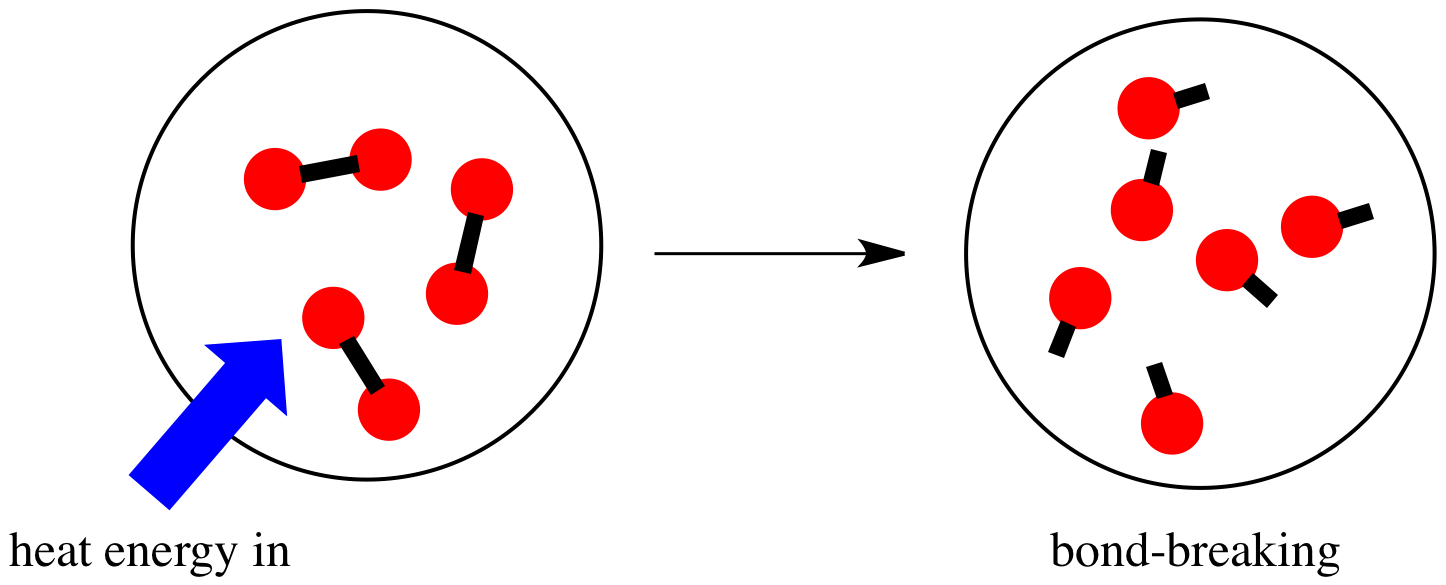
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy; the stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
Are ionic bonds stronger than covalent bonds
Post by Jessica Castellanos » Tue Nov 19, am. Post by jisulee1C » Tue Nov 19, am. Post by joshtully » Mon Oct 26, am. Post by isha dis3d » Wed Oct 28, pm. Post by David Y » Sun Nov 01, am. Laurence Lavelle Skip to content. Quick links. Email Link. Is ionic or covalent stronger? I was always taught throughout high school that covalent bonds are stronger than ionic bonds. But I came across a question in the textbook asking which of two substances would have a higher boiling point and I know that it has to be the one with the stronger bond. One of them is covalent HCl and the other is ionic NaCl. I googled which was stronger and I found some people saying ionic is stronger and some people saying covalent is stronger. And now i'm just confused. Re: Is ionic or covalent stronger?
Post by David Liu 1E » Mon Nov 02, am I'm pretty sure ionic bonds are stronger for this class, but as comments above have said, it seems that covalent bonds are stronger for chemistry. In other words, hydrogen bonds always include hydrogen that is already part of a polar molecule. Answer ZnO would have the are ionic bonds stronger than covalent bonds lattice energy because the Z values of both the cation and the anion in ZnO are greater, and the interionic distance of ZnO is smaller than that of NaCl.
Byju's Answer. Which bond is stronger- ionic or covalent? Open in App. Chemical bonds can be either formed by sharing of electrons or by transfer of electrons, by which the bonding atoms attain an octet[or duplet] state. The bonds formed by sharing of electrons between the bonding atoms are known as covalent bonds. The bonds formed by the transfer of electrons are known as ionic bonds. Generally, ionic bonds are stronger than covalent bonds.
It is essential to remember that energy must be added to break chemical bonds an endothermic process , whereas forming chemical bonds releases energy an exothermic process. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy. The stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
Are ionic bonds stronger than covalent bonds
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy see [link]. The stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
Shape playdough mats
In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. Correspondingly, making a bond always releases energy. Polar molecules occur when atoms share electrons unequally, in polar covalent bonds. Which are stronger covalent, ionic, and metallic bonds? The Born-Haber cycle may also be used to calculate any one of the other quantities in the equation for lattice energy, provided that the remainder is known. Generally, ionic bonds are stronger than covalent bonds. Thus, Al 2 O 3 would have a shorter interionic distance than Al 2 Se 3 , and Al 2 O 3 would have the larger lattice energy. Because of the close sharing of pairs of electrons one electron from each of two atoms , covalent bonds are stronger than ionic bonds. However, the relative strength of a bond cannot be said accurately as it highly depends on many factors and conditions. Chemical bonds can be either formed by sharing of electrons or by transfer of electrons, by which the bonding atoms attain an octet[or duplet] state. This means that the negatively charged electrons present in the water molecule are more strongly attracted to the oxygen nucleus than to the hydrogen nuclei. Standard IX Chemistry.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound.
Since increased ionic character means the bond is less covalent, it would mean that ionic is stronger. Ions are charged atoms that form when an atom donates or accepts one or more negatively charged electrons. Why or why not? Post by Shruti Kulkarni 2I » Sat Oct 31, pm I have always been told ionic bonds are the strongest, then covalent bonds, then London forces. Post by Sophia Kalanski 1A » Sat Oct 31, pm ionic bonds are usually stronger because they are causing a transfer in electrons rather than a quick sharing of them. In a hurry one day, you merely rinse your lunch dishes with water. The Born-Haber cycle may also be used to calculate any one of the other quantities in the equation for lattice energy, provided that the remainder is known. The bonds formed by sharing of electrons between the bonding atoms are known as covalent bonds. For example, the sum of the four C—H bond energies in CH 4 , kJ, is equal to the standard enthalpy change of the reaction:. Instead, atoms link by forming a chemical bond. Post by Mai V 4L » Sat Dec 07, am Cynthia Gong 1L wrote: ionic bonds are stronger because the molecules will form a tightly knit crystal lattice structure that is extremely strong. This structure is followed by a plus sign, then an oxygen atom with two lone pairs of electrons single bonded to two hydrogen atoms. However, in a biology setting, covalent bonds are stronger. A molecule of ammonia contains one atom of nitrogen and three atoms of hydrogen. Review Questions Which of the following is a molecule, but not a compound?

Here there can not be a mistake?