Average atomic mass of sulfur
The natural isotopes of sulfur are listed below: 32 S, Skip to main content. Table of contents. Intro to General Chemistry 0.
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days. Sulfur was known as brenne stone for "combustible stone" from which brim-stone is derived. It was known from prehistoric times and thought to contain hydrogen and oxygen.
Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance , which is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S Chemistry Matter Atomic Mass. Stefan V. Oct 26, Explanation: The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. Related questions How do you calculate the atomic mass of carbon? How are atomic mass and mass number different? How do you calculate atomic mass from isotopic composition? How do atomic mass and atomic weight differ?
Chemistry
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundance , which is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S Chemistry Matter Atomic Mass. Stefan V. Oct 26, Explanation: The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances.
Average atomic mass of sulfur
Sulfur is abundant, multivalent, and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow crystalline solid at room temperature. Chemically, sulfur reacts with all elements except for gold, platinum, iridium, tellurium, and the noble gases. Sulfur is a chemical element with atomic number 16 which means there are 16 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
Brothers in law taphouse menu
Cell Notation. Try it in the Numerade app? Subatomic Particles. Intro to Acid-Base Titration Curves. Get Better Grades Now. Osmotic Pressure. Writing Formulas of Coordination Compounds. Factors Influencing Rates. Face Centered Cubic Unit Cell. Solutions: Mass Percent. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Arrhenius Equation.
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg.
Spatial Orientation of Bonds. Related questions How do you calculate the atomic mass of carbon? Parts per Million ppm. Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. Periodic Trend: Ionization Energy. Speed of Light. Electron Configurations of Transition Metals: Exceptions. So we have our isotopes here. Van der Waals Equation. Chemical Thermodynamics 0. Chemistry of the Nonmetals 0. Wavelength and Frequency. Isomerism in Coordination Complexes. Naming Ketones. How much atomic mass is in hydrogen?

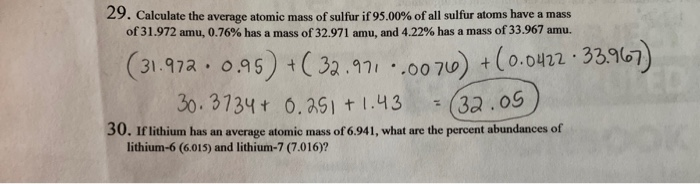
Bravo, the excellent message