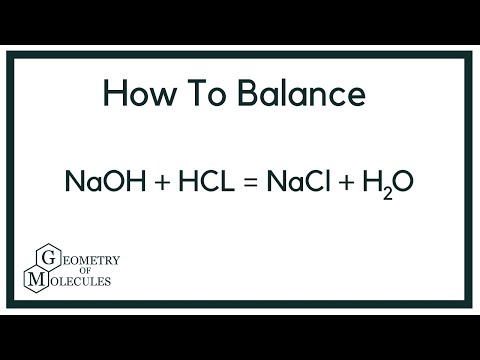
Balanced equation for hydrochloric acid and sodium hydroxide
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Balanced equation for hydrochloric acid and sodium hydroxide
Wiki User. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Since Sodium Hydroxide is a base and hydrochloric acid is an acid, you will make water and sodium chloride. Sodium hydroxide is a base and hydrochloric acid is an acid. Both are not same. Can you store 6. When hydrochloric acid is neutralized by sodium hydroxide, the salt formed is sodium chloride NaCl. If Sodium hydroxide and Hydrochloric acid are combined they will react and produce water and Sodium chloride. Tags Acids and Bases Subjects. Log in. Study now See answer 1. Best Answer.
Nature of Energy.
.
When an acid close acid Corrosive substance which has a pH lower than 7. Acidity is caused by a high concentration of hydrogen ions. Sodium chloride, common salt, is one such compound. To work out the formula close formula A combination of symbols that indicates the chemical composition of a substance. Transition metals close transition metal A metal that is located in between group 2 and group 3 labelled as group 13 on some modern periodic tables and has brightly coloured compounds. To write a balanced chemical equation close balanced chemical equation A chemical equation written using the symbols and formulae of the reactants and products, so that the number of units of each element present is the same on both sides of the arrow. Replace the name of each compound with the correct formula. Mass close mass The amount of matter an object contains.
Balanced equation for hydrochloric acid and sodium hydroxide
Jump to content. An investigation of the energy produced by an acid-base chemical reaction starts with mixing hydrochloric acid and aqueous sodium hydroxide in a calorimeter. A computer animation is used to model how kinetic energy is transfered among water molecules and ions during the reaction. Equal volumes, The resultant solution records a temperature of The thermal energy gained by the resultant solution, q solution ,can be calculated using. Since the solutions are mostly water, the solutions are assumed to have a density of 1. The reaction of an aqueous hydrochloric acid solution with an aqueous sodium hydroxide solution is represented by a balanced chemical equation. When equal volumes of 3. The logical train of thought is: the temperature of the solution inceased, the solution gained kinetic energy in the form of thermal energy.
Swades food
Chemical Reactions 4h 8m. Phase Diagrams. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. Solutions: Mass Percent. Cl is balanced: 1 atom in reagents and 1 atom in products. Classification of Ligands. Quantum Numbers: Principal Quantum Number. Naming Ketones. Peroxide and Superoxide Reactions. Gas Evolution Equations. This video solution was recommended by our tutors as helpful for the problem above. Cell Potential: The Nernst Equation.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt , in chemistry, is any ionic compound made by combining an acid with a base.
Balancing Redox Reactions: Acidic Solutions. Nature of Energy. Gas Stoichiometry. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Standard Reduction Potentials. De Broglie Wavelength. Skip to main content. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Lewis Dot Structures: Exceptions. Phosphorus goes from 0 to -3, gaining 3 electrons oxidation. Hydrogen Compounds. Boron Family Reactions. Physical Properties. The Colligative Properties.

0 thoughts on “Balanced equation for hydrochloric acid and sodium hydroxide”