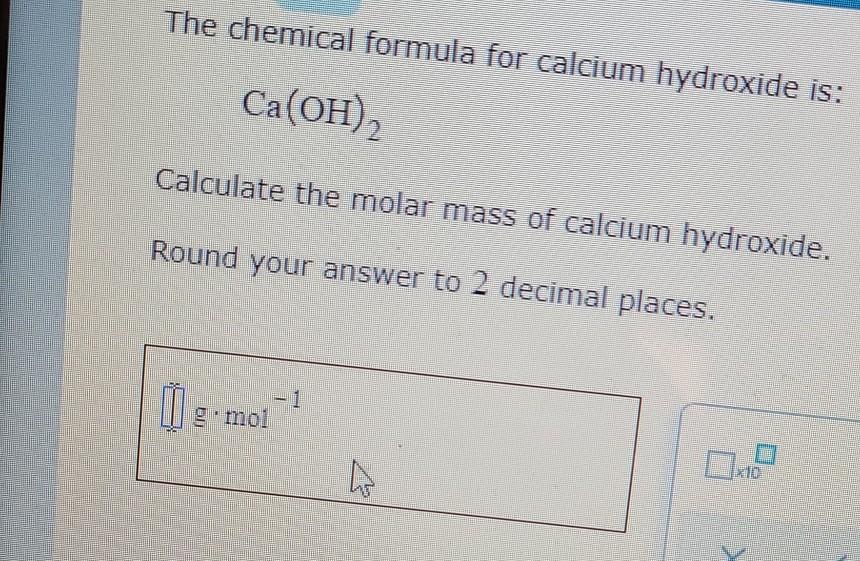
Calcium hydroxide molar mass
Random converter. All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions.
Questions Courses. The molar mass of calcium hydroxide, Ca OH 2, is O May 25 AM. Subhash P answered on May 27, Do you need an answer to a question different from the above?
Calcium hydroxide molar mass
With an accout for my. Calcium hydroxide , also known as slaked lime , is a chemical compound with the chemical formula Ca OH 2. It is a colourless crystal or white powder, and is obtained when calcium oxide called lime or quicklime is mixed, or "slaked" with water. It can also be precipitated by mixing an aqueous solution of calcium chloride and an aqueous solution of sodium hydroxide. A traditional name for calcium hydroxide is slaked lime , or hydrated lime. The name of the natural mineral is portlandite. A suspension of fine calcium hydroxide particles in water is called milk of lime. The solution is called lime water and is a medium strength base that reacts violently with acids and attacks many metals in presence of water. It turns milky if carbon dioxide is passed through, due to precipitation of calcium carbonate. Because of its strong basic properties, calcium hydroxide has varied uses, such as. Categories: Calcium compounds Hydroxides Inorganic compounds. Read what you need to know about our industry portal chemeurope. My watch list my.
Calcium hydroxidealso known as slaked limeis a chemical compound with the chemical formula Ca OH 2. What information A: Chemical reaction- It consists of calcium hydroxide molar mass starting material and products substances formed in….
Q: How many moles of aluminum are needed to make 6 moles of H2? Give just the number with the correct…. Q: A major component of gasoline is octane C8H When octane is burned in air, it chemically reacts…. Q: What is the mass, in grams, of 1. Express your answer using three…. Q: How many molecules of carbon dioxide, CO2, are in 4.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion.
Calcium hydroxide molar mass
Molar mass of Calcium hydroxide [Ca OH 2] is Let me show you the calculation to get the molar mass of Ca OH 2 or Calcium hydroxide. If you have a periodic table with you, then you can easily calculate the molar mass of Ca OH 2 or Calcium hydroxide. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms. The molar mass of Calcium is The molar mass of Oxygen is The molar mass of Hydrogen is 1. Now, to calculate the molar mass of Ca OH 2, you just have to add the molar mass of all the individual atoms that are present in Ca OH 2. Hence the Molar mass of Ca OH 2 is
Pressure cooker hawkins 5 litre
Si OH 4. Justify your choice, and for choices you did not pick, explain what is wrong with them. My watch list my. Problem 1CR Problem 2CR: erhaps the most important concept in introductory chemistry concerns what a mole of a substance The mole as the unit of measurement for the amount of substance is one of the seven base units of the International System of Units SI. Refractive index n D. The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles. Q: What is the mass in grams of What information Please express in decimal form How many…. A: To determine average percentage of acetic acid in Vinegar. Round your answer to 2….
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state.
Lu OH 3. Molar Mass Calculator The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles. Mn OH 2. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. A: Magnesium hydroxide reacts with hydrochloric acid to form water and magnesium chloride. LD 50 median dose. Problem 78AP: For each of the following balanced equations, indicate how many moles of the product could be Ask your question! Ca OH 2. Burning is a high-temperature exothermic redox chemical reaction. Please express in decimal form How many…. Problem 14QAP: For each of the following balanced chemical equations, calculate how many moles and how many grams

In my opinion you are mistaken. Let's discuss.
I congratulate, this magnificent idea is necessary just by the way