Ch3cn lewis structure
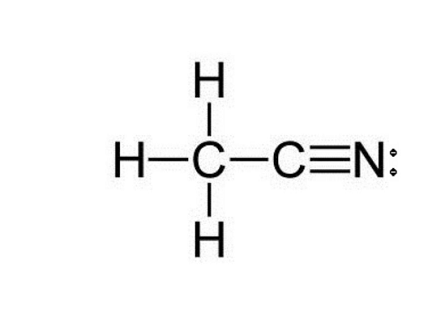
There is a triple bond between the Carbon atom C and Nitrogen atom N. There is 1 lone pair on the Nitrogen atom N, ch3cn lewis structure. In order to find the ch3cn lewis structure valence electrons in a CH3CN moleculefirst of all you should know the valence electrons present in carbon atomhydrogen atom as well as nitrogen atom.
Wiki User. The C on the left has three singly bonded H atoms, and the right C has a triple bonded N atom that has one pair of double dots. It is also connected to another oxygen by single bond with 3 pairs of electrons this oxygen has a negative charge. Ethanenitril or acetonitrile. Lewis structure was created in
Ch3cn lewis structure
For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen, giving us a total of 16 valence electrons. Carbon's the least electronegative, so that's going to go at the center. We'll put the other Carbon here and then the Nitrogen on this side. We can tell by the way it's written, that the CH3 means we're going to have Hydrogens around this Carbon right here, and the Nitrogen will be here on the other side. So we have 3 Hydrogens around this Carbon here. We have our central Carbon, and then we have our Nitrogen over here. We'll put 2 electrons between atoms to form chemical bonds. We've used 6, 8, 10; and then around the outside, 12, 14, and we've used all 16 valence electrons right now that we started with. Remember, Hydrogen only needs 2 for a full outer shell. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. Now Nitrogen still has 8 but Carbon has six, so we're getting close to an octet for Carbon. Let's move 2 more electrons here and share them with the Carbon. By forming that triple bond, we see that Nitrogen has 8 valence electrons and the Carbon has 8 valence electrons. We've only used 16 valence electrons that we started with.
For selecting the center atom, you have to ch3cn lewis structure that the atom which is less electronegative remains at the center. We'll put 2 electrons between atoms to form chemical bonds. See the Big List of Lewis Structures.
Acetonitrile, also known as methyl cyanide is a colorless organic liquid with an aromatic odor. It is majorly produced as a byproduct during the manufacturing of acrylonitrile. It is used in the organic synthesis of many compounds where it acts as a polar aprotic solvent. It was first produced by Jean Baptiste Dumas in It is also a potent air pollutant found in automobile and industrial exhausts. In this article, we will study the lewis structure of CH3CN along with its geometry, hybridization, and polarity.
Acetonitrile, also known as methyl cyanide is a colorless organic liquid with an aromatic odor. It is majorly produced as a byproduct during the manufacturing of acrylonitrile. It is used in the organic synthesis of many compounds where it acts as a polar aprotic solvent. It was first produced by Jean Baptiste Dumas in It is also a potent air pollutant found in automobile and industrial exhausts. In this article, we will study the lewis structure of CH3CN along with its geometry, hybridization, and polarity. Read till last. Lewis dot symbol or lewis structures are the diagrams that demonstrate the bonding between different atoms of a compound, specifying the number of bonds as well as the lone pairs of electrons.
Ch3cn lewis structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
Hull c star citizen
As per VSEPR theory, the three-dimensional shape of any molecule is determined by these inter-electronic repulsion forces working inside the molecule. Also, a lone pair of electrons is left with the nitrogen atom. Lewis structure for CH2N2. Do XeF4 have a Lewis structure? Potassium oxide K2O is an ionic compound, not a molecule, and does not have a Lewis structure. This step enables us to estimate the number of electrons that are still required by one or more atoms of the molecule to complete their octet. November 23, It is an ionic compound so it would not have a Lewis dot structure. I'm Dr. Therefore, it is the correct lewis structure. The hybridization of any molecule is determined by calculating the steric number for that molecule. By forming that triple bond, we see that Nitrogen has 8 valence electrons and the Carbon has 8 valence electrons. These forces are maximum between the lone pair-lone pair as these electrons are free to move in space while minimum between the bond pair-bond pair. We already know that in an atom, electrons revolve around the nucleus in definite orbits that are known as shells.
The Lewis structure of CH 3 CN contains four single bonds and one triple bond, with two carbons in the center. The left carbon is attached with three hydrogens, and the right carbon is attached with one nitrogen. There is one lone pair on the nitrogen atom, and carbon atom and hydrogen atom do not have any lone pair.
Which is more polar acetonitrile or methnol? He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. The lewis structure of a molecule helps us determine the number of bond pairs and lone pairs present in that molecule. It was first produced by Jean Baptiste Dumas in In short, now you have to find the formal charge on hydrogen H atom, carbon C atom as well as nitrogen N atoms present in the CH3CN molecule. Ethanenitril or acetonitrile. The acetonitrile molecule is polar owing to the difference in the electronegativity of carbon and nitrogen atom due to which a slight negative charge develops on nitrogen while a slight positive charge on the carbon atom. It is assumed that an atom tends to form bonds in order to attain stability. Therefore, the electrons inside a molecule repel away from each other. Also, in step 1 we have calculated the total number of valence electrons present in the CH3CN molecule. We already know that all the electrons carry a negative charge and also that like charges repel each other. In this case, the electron geometry of the molecule is also tetrahedral and the bond angle between different atoms is What is the hybridization of acetonitrile? We've used 6, 8, 10; and then around the outside, 12, 14, and we've used all 16 valence electrons right now that we started with. The stability of lewis structure can be checked by using a concept of formal charge.

0 thoughts on “Ch3cn lewis structure”