Charge of io3
Iodate ion contains one iodine and three oxygen atoms.
IO 3 — lewis structure has an Iodine atom I at the center which is surrounded by three Oxygen atoms O. There is 1 single bond and 2 double bonds between the Iodine atom I and each Oxygen atom O. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. In order to find the total valence electrons in IO3- ion, first of all you should know the valence electrons present in iodine atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Iodine is a group 17 element on the periodic table. Oxygen is group 16 element on the periodic table.
Charge of io3
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite : [1]. Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Iodate is unusual in that it forms a strong hydrogen bond with its parent acid : [2]. Minerals containing iodate are found in the caliche deposits of Chile. Contents move to sidebar hide. Article Talk.
Polyatomic anion IO3 with charge So the above lewis dot structure of IO3- ion can also be represented as shown below.
Wiki User. Rubidium iodate. Formula: NaIO3. Pt IO3 8. Formula: CuIO3. The chemical formula of iodate is IO It consists of one iodine atom bonded to three oxygen atoms.
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of IO Here, the given ion is IO In order to draw the lewis structure of IO3- ion, first of all you have to find the total number of valence electrons present in the IO3- ion. Valence electrons are the number of electrons present in the outermost shell of an atom.
Charge of io3
Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. Usually, the name of polyatomic cations ends with —ium, and the name of polyatomic anions end with —ide, except for oxyanions that have separate rules for their nomenclature. The oxyanions are oxides of nonmetals that are molecular ions. The following guidelines will help remember the names and charges of oxyanions in most cases. Oxyanins of chlorine, bromine, and iodine are also common oxyanions with the following in common. Names of the acids are the names of oxyanions with -ate replaced with -ic acid and -ite replaced with -ous acid. The prefixes "per-" and "hypo-" in the cases of oxyanions of halogens remain in the acid name.
Yale.mychart
Now, in the above structure, you can see that the charges are minimized and the above lewis structure of IO3- is the final stable structure. We really want our formal charges to be as close to zero as possible. In short, now you have to find the formal charge on iodine I atom as well as oxygen O atoms present in the IO3- ion. Therefore it can hold more than 8 valence electrons. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. About Iodates Iodates are a class of iodine -containing chemical compounds analogous to the chlorine-containing chlorates. Jay Rana. In the above lewis dot structure of IO3- ion, you can also represent each bonding electron pair : as a single bond. Share This Page. This overall -1 charge on the IO3 molecule is represented in the image given below.
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom.
You can see the electronegativity values of iodine atom I and oxygen atom O in the above periodic table. It consists of one iodine atom bonded to three oxygen atoms. Space-filling model of the iodate anion. Additionally, iodates that do not contain toxic metals, such as potassium iodate or calcium iodate , can be used for iodine supplements or radioactivity prophylaxis treatments. Rubidium iodate. See the Big List of Lewis Structures. CAS Number. Formula: CuIO3. Therefore, we should try to reduce charges by converting lone pairs to bonds to find the most stable structures. Remember that, there are total of thirteen electron pairs. Resources Leaderboard All Tags Unanswered. Because IO 3 - ion is an ion and there are four atoms, we will have more steps than drawing a simple molecule. Number of steps can be changed according the complexity of the molecule or ion. In order to draw the lewis structure of IO3- ion, first of all you have to find the total number of valence electrons present in the IO3- ion. In simple words, we have to check whether the central Iodine I atom is having 8 electrons or not.

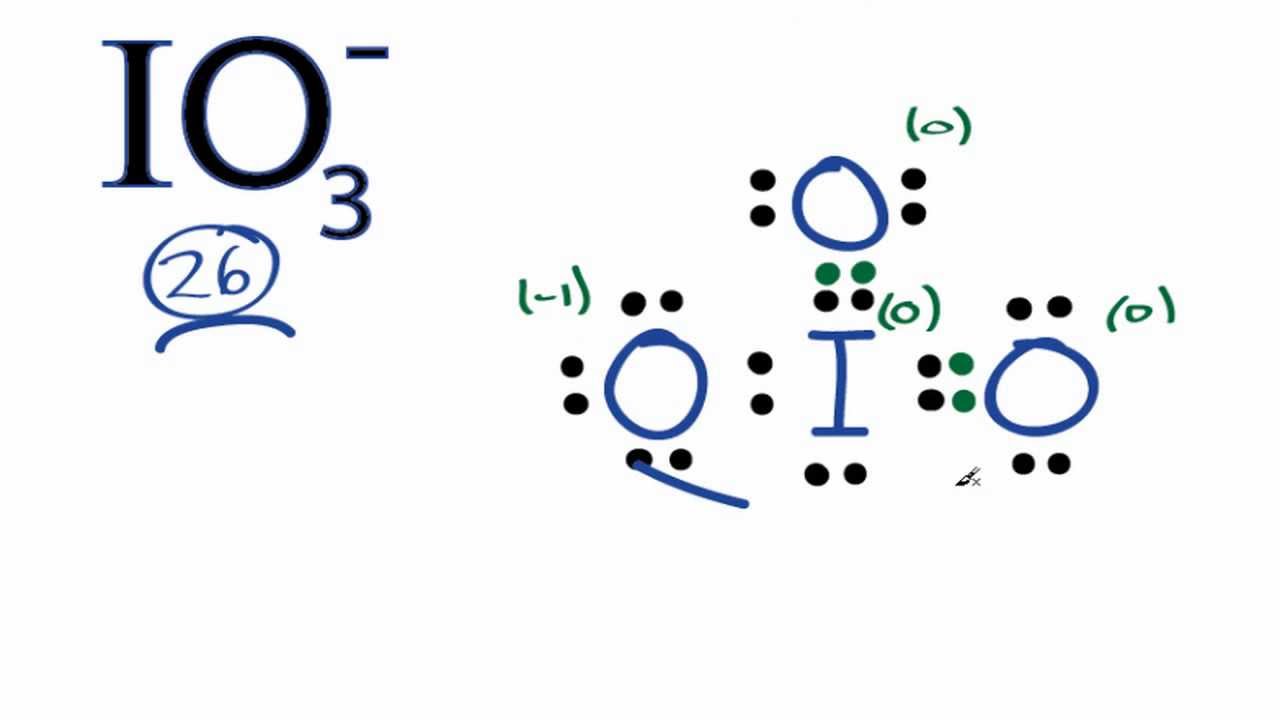
0 thoughts on “Charge of io3”