Chlorine bohr model
The two electrons in the first shell should be together not single. This is a Bohr model of a chlorine atom.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n.
Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye and even most microscopes. So, we represent atoms using models. Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms. However, it's important to remember that no scientific model is perfect. Every model sacrifices some accuracy for simplicity, visibility, or usability. If a model was perfect, it wouldn't be a model—it would be the real thing!
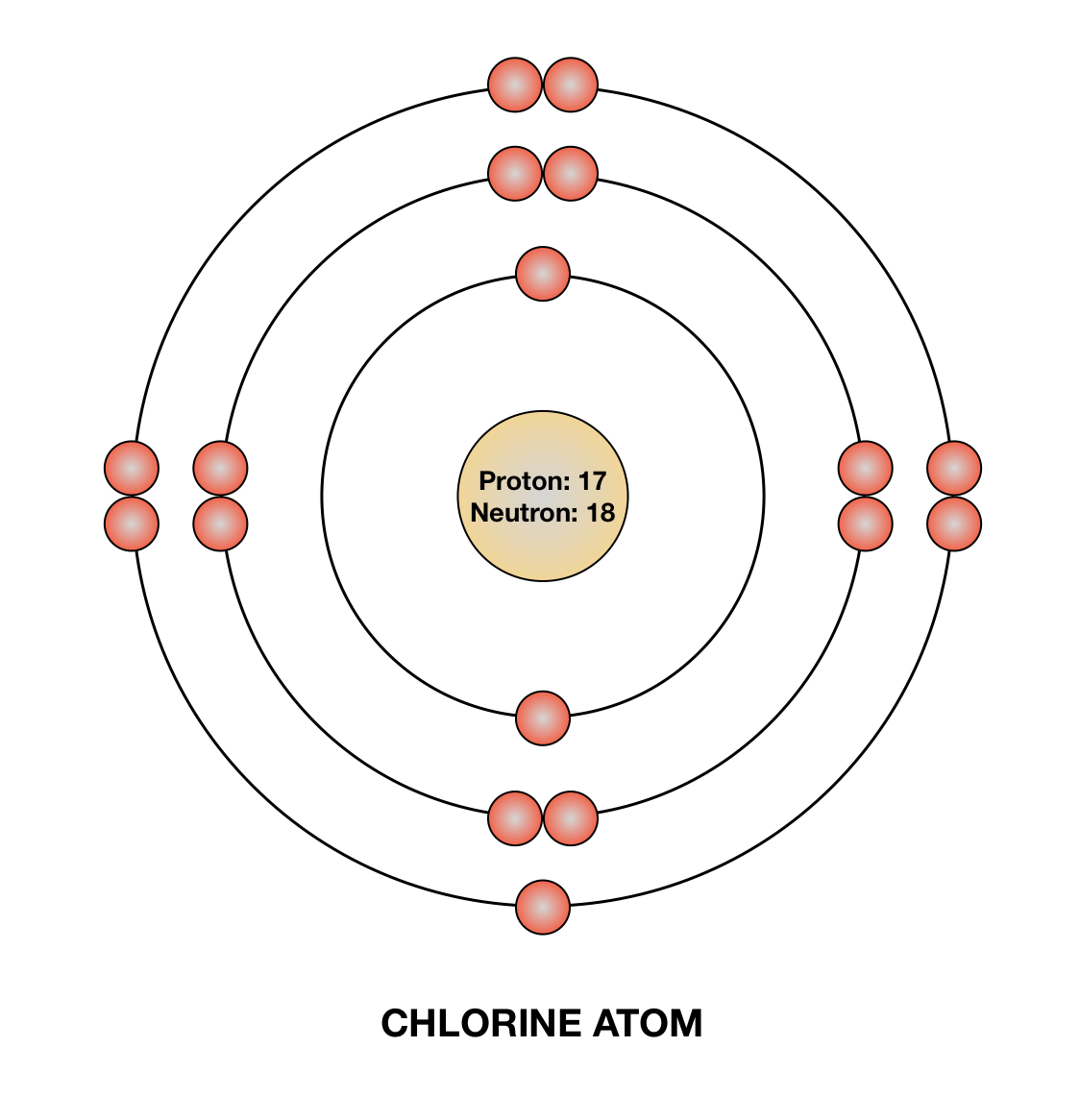
The ring closest to the chlorine bohr model contains 2 black dots, the second ring from the nucleus contains 8 black dots, and the third ring from the nucleus contains 7 red dots. Bohr Diagrams Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Quantum mechanics is weird!
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Virginia McDaniel Modified over 5 years ago. They become negatively charged because there will be more electrons than protons.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye and even most microscopes. So, we represent atoms using models. Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms. However, it's important to remember that no scientific model is perfect.
Chlorine bohr model
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
Ford f850
K shell can have 2, L can have 8 , M can have 18 electrons and so on. Tools Tools. As a result of losing a negatively-charged electron, they become positively-charged ions. To make this website work, we log user data and share it with processors. A negative ion will be formed. Remember… Atoms combine by gaining, losing or sharing electrons in order to become chemically stable. The best we can do is describe where we're likely to find electrons around a nucleus. Bohr models of the following elements are shown, with the corresponding numbers of electrons e , shown as dots, in each shell. This is a fundamental property of quantum mechanics. Unlike when we consider an infinite range of imaginary values between in mathematics, this isn't the case at a quantum level, where there is no in between. Models help us visualize atomic structure. Bohr models of some elements from the first three rows periods of the periodic table are shown below. The energy levels in a Bohr model are also called shells. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. If you wish to download it, please recommend it to your friends in any social system.
The Bohr model of chlorine contains a nucleus having 17 protons and 18 neutrons in the center, and around this nucleus, there are three electron shells containing 17 electrons.
Show preview Show formatting options Post answer. If you're seeing this message, it means we're having trouble loading external resources on our website. We can tell that the two electrons in the model above are at the same energy level because they are on the same ring. Similarly, neon has a complete outer 2n shell containing eight electrons. A full valence shell is the most stable electron configuration. The Bohr model represents these energy levels as rings. Bohr diagrams Bohr diagrams indicate how many electrons fill each principal shell. Every model sacrifices some accuracy for simplicity, visibility, or usability. Why did I read somewhere that some atoms are different and they dont follow this Octet Rule and their third shell can hold up to 18 electrons? We think you have liked this presentation.

0 thoughts on “Chlorine bohr model”