Compound containing both ionic and covalent bonds
Last updated on Jan 7, Get Started.
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bonds , on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions. Many of these compounds contain a metal, a nonmetal, and also hydrogen. However, other examples contain a metal joined via an ionic bond to covalently bonded nonmetals. Here are examples of compounds that exhibit both types of chemical bonding:.
Compound containing both ionic and covalent bonds
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involves atoms sharing electrons. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. For example, in potassium cyanide KCN , the carbon C and nitrogen N are both nonmetals, so they share a covalent bond.
Bihar Police SI.
.
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom.
Compound containing both ionic and covalent bonds
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation a positive ion forms when a neutral atom loses one or more electrons from its valence shell, and an anion a negative ion forms when a neutral atom gains one or more electrons in its valence shell. Compounds composed of ions are called ionic compounds or salts. The ions are held together by ionic bonds: electrostatic forces of attraction between oppositely charged cations and anions. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle. They also tend to have high melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid state.
Love never dies synopsis
UP PGT. Bank of Baroda PO. RRB Office Assistant. Cod liver oil obtained from fish is rich in:. What is another name of quick lime? ITBP Constable. Maharashtra Zilla Parishad Rigman. Get Started. Maharashtra Zilla Parishad Extension Officer. Puducherry Fireman. Odisha Police Constable. TN Forest Guard. Tripura TET.
In chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged.
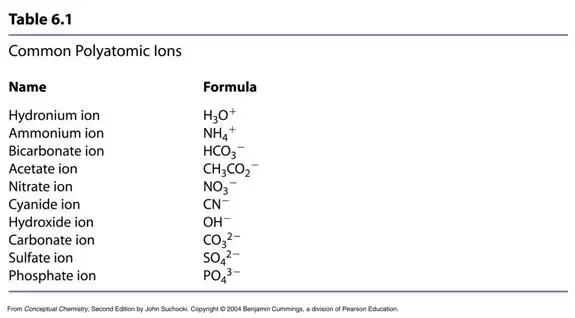
These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. FCI Watchman. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. West Bengal Police Warder. AOC Tradesman Mate. Bihar CET B. Chandigarh Police Constable. WRD Maharashtra. CIL MT. Bombay High Court Clerk. By Anne Marie Helmenstine, Ph. Patna Civil Court Clerk.

I know a site with answers to a theme interesting you.
It seems remarkable idea to me is