Daniell cell class 12
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger?
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project.
Daniell cell class 12
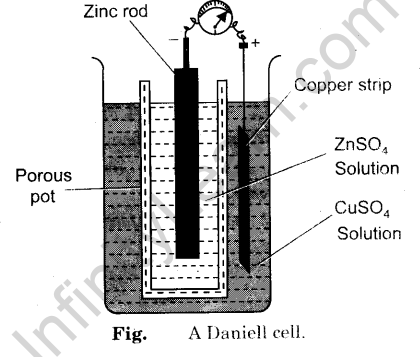
Byju's Answer. Explain Daniel cell with cell diagram representation and process taking place in the cell. Open in App. Daniel Cell Daniel cell is an electrochemical device that can convert chemical energy to electrical energy. In an electrochemical cell, the anode is negative and the cathode is positive. Electrons flow from anode to cathode. In the daniel cell, Anode is made of Zinc Zn metal dipped in Zinc salt solution i. Cell Diagram At the anode, oxidation takes place. The double vertical lines represent the salt bridge. Working principle The oxidation of Zinc metal at the anode produces 2 electrons.
Save Myself Save Myself. The half-cells are joined by a salt bridge that prevents the mechanical mixing of the solution.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell. In this case, a saturated CuSO 4 solution is used as a depolarizer and diluent. Fill with H 2 SO 4 , which works as an electrolyte. Zn 2 SO 4 is used to submerge a zinc rod that has been amalgamated.
Daniell cell class 12
A Daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulphate and copper sulphate respectively. A typical galvanic cell, it is designed to make use of the spontaneous redox reaction between zinc and cupric ion to produce an electric current. This cell consists of a copper vessel. In which saturated CuSO 4 solution is filled which acts as depolarizer and dil. H 2 SO 4 is filled which acts as an electrolyte. An amalgamated zinc rod is immersed in Zn 2 SO 4. In copper vessels there is a transparent layer all around just below the upper surface in which CuSO 4 crystals are kept in contact with CuSO 4 solution due to this the solution always remains saturated. It maintains electrical neutrality in two compartments by allowing movement of anions towards anodic compartment and cations towards cathodic compartment. It is known as cell Daniell.
Youtube 30 million subscribers play button
Usually, for demonstration in classrooms, a form of Daniell cell also called the two-half cells is used to simplify the explanation. Instead, a layer of zinc sulfate sits on top of a layer of copper sulfate, the two liquids are kept separate by their differing densities, often with a layer of oil added on top to prevent evaporation. Personal Growth Documents. In a Daniell cell, metal ions move from one half of the cell to the other through the salt bridge while electrons move from the zinc electrode to the copper electrode through an external circuit. Copper ions are reduced to copper metal. The copper vessel was filled with sulfuric acid solution saturated with copper sulfate to above the level of the perforated disc. The ox-gullet acts as a porous membrane allowing passage of ions. The replacement of sulfuric acid with zinc sulfate was the innovation of J. You can rewrite your notes as well if you find them a bit messy. Application It is used to produce electricity. According to the chemical reaction occurring at them, the anode in an electrochemical cell is known as the oxidation half cell and the cathode as the reduction half cell. Question 3. Thanks for Byjus.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions.
Chemistry Chemistry. When the two metal electrodes are connected with help of an external circuit, the copper electrode attracts the electrons leftover in the zinc as the metal oxidises in zinc sulphate. A galvanic cell is a type of electrochemical cell that utilises electrical energy produced by natural r Bird's experiments with this cell were of some importance to the new discipline of electrometallurgy , but Bird himself did not pursue this field; his interest was in electrotherapy. Leave a Reply Cancel reply Your email address will not be published. T for providing us with a well equipped laboratory for carrying out our experiments. Filip Filip. A wire and light bulb may connect the two electrodes. Over time, copper buildup will block the pores in the earthenware barrier and cut short the battery's life. The ox-gullet tube was filled with sulfuric acid solution. For every pair of electrons of zinc that goes to the copper electrode, one sulphate ion passes through the solution for compensation from the copper side through the porous earthenware immersed in the dilute sulphuric acid.

In my opinion, you are mistaken.
In it something is. Thanks for an explanation.