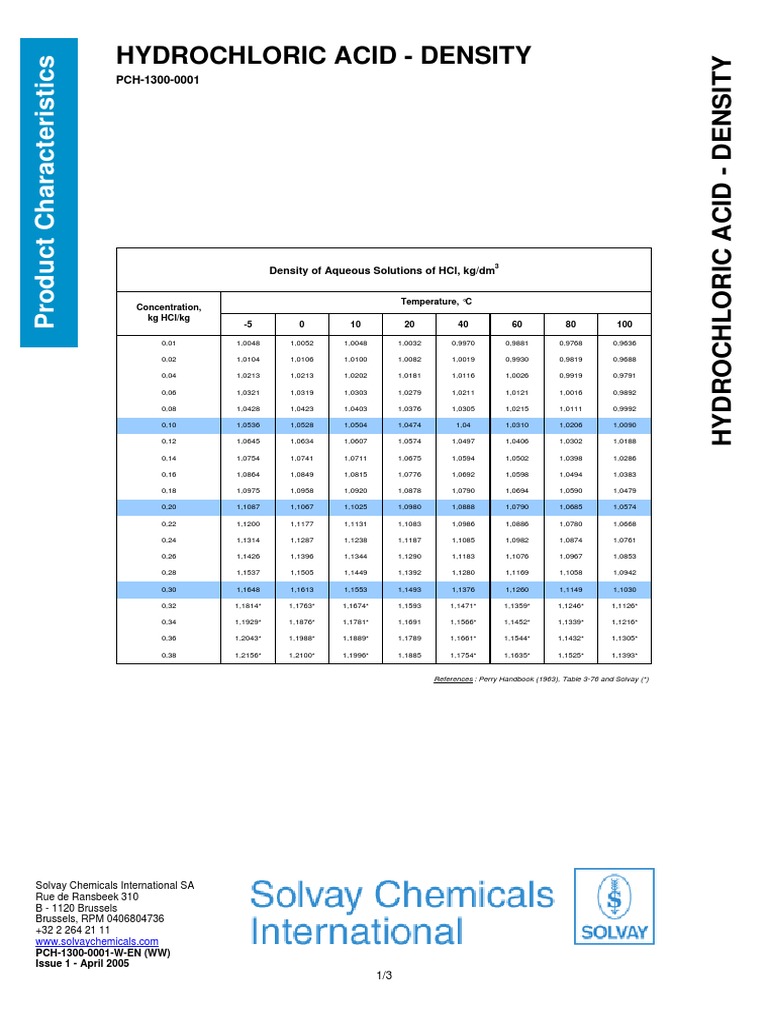
Density of hcl solutions
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, density of hcl solutions, such as polyvinyl chloride for plastic.
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. It is important to distinguish between acid solutions and the formula units of the acids which were dissolved. Examine the table below. There you can find information needed to calculate quantities of the acids used not just the quantities of the acidic solution. You can cite the Lab Manual as the source for these data.
Density of hcl solutions
Steffen's Chemistry Pages. Return to Density tables. Average rating 4. Vote count: No votes so far! Be the first to rate this post. Made with by Graphene Themes. Toggle search form Search for:. Toggle navigation Steffen's Chemistry Pages Facts and tables around chemistry. Home » Chemistry » Density tables » Density of hydrochloric acid. Click on a star to rate it! Post Views: 38, Leave a Reply Cancel reply. In this section Density tables Density of Ammonia Density of Dry Air Density of hydrochloric acid Density of nitric acid Density of perchloric acid Density of phosphoric acid Density of potassium hydroxide Density of sodium hydroxide Density of some gases Density of sulfuric acid and sulfur trioxide Density of Water Ethanol- Water- Mixtures. Random Quote A brave spirit struggling with adversity is a spectacle for the gods.
The first clear instance of the preparation of hydrochloric acid appears in the writings of Della Porta, andLibaviuspseudo-Basilvan Helmont and Glauber Diastase Pancreatin Pancrelipase Pepsin. Salts and covalent derivatives of the chloride ion.
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air.
In preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances. Similar to a pure substance, the relative composition of a mixture plays an important role in determining its properties. The relative amount of the active ingredient in a medicine determines its effectiveness in achieving the desired pharmacological effect. In this section, we will describe one of the most common ways in which the relative compositions of mixtures may be quantified. We have previously defined solutions as homogeneous mixtures, meaning that the composition of the mixture and therefore its properties is uniform throughout its entire volume. Solutions occur frequently in nature and have also been implemented in many forms of manmade technology. We will explore a more thorough treatment of solution properties in the chapter on solutions and colloids, but here we will introduce some of the basic properties of solutions. The relative amount of a given solution component is known as its concentration.
Density of hcl solutions
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:. Data compiled as indicated in comments: B - John E.
Escort olesa de montserrat
Hydrochloric acid is an important laboratory reagent and industrial chemical. Return to Density tables. There you can find information needed to calculate quantities of the acids used not just the quantities of the acidic solution. Since hydrochloric acid was already fully settled as an important chemical in numerous applications, the commercial interest initiated other production methods, some of which are still used today. Vapors or mists are a respiratory hazard, which can be partially mitigated by use of a respirator equipped with cartridges specifically designed to capture hydrochloric acid. To convert mass to moles, we need the molecular weight. January The spent acid has long been reused as iron II chloride also known as ferrous chloride solutions, but high heavy-metal levels in the pickling liquor have decreased this practice. The resulting hydrogen chloride gas is absorbed in deionized water , resulting in chemically pure hydrochloric acid. DyCl 2 DyCl 3. Hydrochloric acid is used for a large number of small-scale applications, such as leather processing, household cleaning, [35] and building construction. In aqueous solutions dissociation is complete, with the formation of chloride ions and hydrated hydrogen ions hydronium ions. SmCl 2 SmCl 3. By recuperation of the spent acid, a closed acid loop is established.
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell.
Thirteenth-century Latin alchemists, for whom the De aluminibus et salibus was one of the main reference works, were fascinated by the chlorinating properties of corrosive sublimate, and they soon discovered that when the metals are eliminated from the process of heating vitriols, alums , and salts, strong mineral acids can directly be distilled. Toggle limited content width. Vapors or mists are a respiratory hazard, which can be partially mitigated by use of a respirator equipped with cartridges specifically designed to capture hydrochloric acid. OCLC Philadelphia: University of Pennsylvania Press. The technical storage or access is necessary for the legitimate purpose of storing preferences that are not requested by the subscriber or user. They do this by tracking which websites visitors go to. As the reaction is exothermic , the installation is called an HCl oven or HCl burner. Some of them are necessary e. Functional Functional Always active The technical storage or access is strictly necessary for the legitimate purpose of enabling the use of a specific service explicitly requested by the subscriber or user, or for the sole purpose of carrying out the transmission of a communication over an electronic communications network. Please cite me: St. Danger [6]. Other anions.

Bravo, what necessary words..., a brilliant idea