Dinitrogen tetrahydride
Submitted by Parker P.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.
Dinitrogen tetrahydride
E-mail: ynishiba sogo. A dinitrogen-bridged dimolybdenum-tetrachloride complex is prepared and reduced with Super-Hydride LiBHEt 3 to afford the corresponding dimolybdenum-dinitrogen complex together with the formation of molecular dihydrogen. This reaction proceeds via the ligand exchange of the coordinated dihydrogen generated in situ with molecular dinitrogen. As the next stage of the previous work, we have focused on the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen at ambient temperature and pressure. To achieve the catalytic formation of ammonia as the next nitrogen fixation, in place of the Haber—Bosch process, 7 the ruthenium—hydride species should reduce the high oxidative tungsten species to regenerate the corresponding tungsten—dinitrogen complex. However, unfortunately, the tungsten species can not be reduced with the ruthenium—hydride species or with other hydride species such as LiBHEt 3. As the first stage of the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen under mild reaction conditions, we envisaged the reaction of the high oxidative molybdenum complexes with hydride species to regenerate the corresponding dinitrogen complexes as starting catalytic species. In this reaction, the high oxidative molybdenum complexes can be reduced into the corresponding dinitrogen complexes, where the ligand exchange of the coordinated dihydrogen with molecular dinitrogen may be involved as a key step to regenerate the corresponding dinitrogen complexes. Herein, we describe the preparation of the dinitrogen-bridged dimolybdenum-tetrachloride complex bearing a PNP -type pincer ligand and the reduction of the dimolybdenum-tetrachloride complex with Super-Hydride LiBHEt 3 to afford the corresponding molybdenum-dinitrogen complex together with the formation of molecular dihydrogen. No informative data on the structure of 2 were obtained from its NMR spectra due to the paramagnetism. A more detailed molecular structure of 2 is determined by X-ray crystallographic study Fig. The molecular structure of 2 contains two [MoCl 2 PNP ] moieties bearing two chloride ligands in trans form.
Find UBC Chemistry on.
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway? If this could be achieved, it would be considered a major breakthrough in this area.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons.
Dinitrogen tetrahydride
This chapter describes the activation of dinitrogen by various transition metal hydride complexes. A number of mononuclear transition metal hydride complexes can incorporate dinitrogen, but they are usually difficult to induce N—N bond cleavage. In contrast, multimetallic hydride complexes can split and hydrogenate dinitrogen through cooperation of the multiple metal hydrides. In this transformation, the hydride ligands serve as the source of both electron and proton, thus enabling the cleavage and hydrogenation of dinitrogen without extra reducing agents and proton sources. This is a preview of subscription content, log in via an institution.
Caras pintadas halloween mujer
Related nitrogen oxides. Wikimedia Commons has media related to Dinitrogen tetroxide. The two molybdenum fragments are twisted around the Mo—N—N—Mo axis with respect to each other away from the steric interaction between two PNP ligands: the torsion angle for Cl 1 —Mo 1 —Mo 2 —Cl 3 is The anhydrous nitrates concerned are themselves covalent, and many, e. It should have been just silicon tetrachloride 25 The name a student gives for the molecular compound SiCl4 is monosilicon trichloride. Retrieved 1 May What is the total of all of the numbers in front of the compounds? About project SlidePlayer Terms of Service. Interactive image. Snapsolve any problem by taking a picture. This synthesis is practical in a laboratory setting. N verify what is Y N? Pedro Paulet, sabio multidisciplinario in Spanish. The gas is essentially pure nitrogen dioxide, which is condensed into dinitrogen tetroxide in a brine-cooled liquefier. Your vid's got the perfect mix of knowledge and clarity!
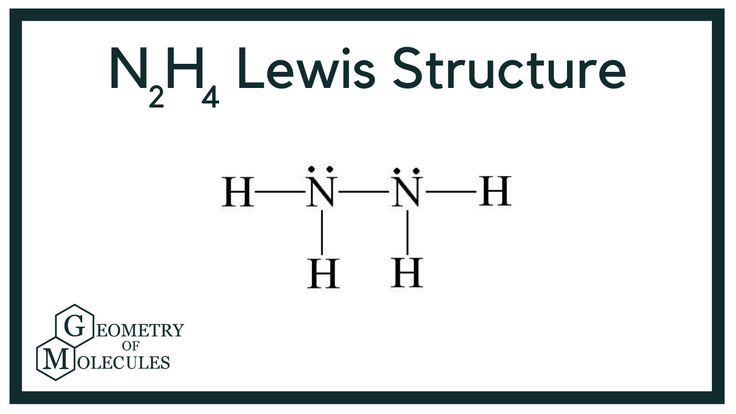
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms. In its anhydrous form, it is a colourless, toxic irritant and sensitiser, which damages the central nervous system, producing symptoms as extreme as tumours and seizures. The pungent smell of hydrazine is not unlike that of ammonia, and it is so powerful a reducing agent that it is highly explosive.
It should have been just silicon tetrachloride 25 The name a student gives for the molecular compound SiCl4 is monosilicon trichloride. S2CID EC Number. Scheme 1 Preparation of a dinitrogen-bridged dimolybdenum-tetrachloride complex bearing PNP pincer ligand 2. Download ppt "dinitrogen tetrahydride". The molecular structure of 2 contains two [MoCl 2 PNP ] moieties bearing two chloride ligands in trans form. Chemical compound. These compounds are just reacted and product. This branch of chemistry was developed by Cliff Addison and Norman Logan at the University of Nottingham in the UK during the s and s when highly efficient desiccants and dry boxes started to become available. OCLC It forms an equilibrium mixture with nitrogen dioxide. Gmelin Reference. View More Comments. Download as PDF Printable version.

Now all became clear, many thanks for the information. You have very much helped me.
There is something similar?