Diphosphate
A number of pyrophosphate salts exist, such as disodium pyrophosphate Na diphosphate H 2 P 2 O 7 and tetrasodium pyrophosphate Na 4 P 2 O 7among others, diphosphate.
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'diphosphate. Send us feedback about these examples. Accessed 9 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! See Definitions and Examples ». Log In. Examples of diphosphate in a Sentence.
Diphosphate
Adenosine diphosphate ADP , also known as adenosine pyrophosphate APP , is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. AMP contains one fewer phosphate group. The cleavage of a phosphate group from ATP results in the coupling of energy to metabolic reactions and a by-product of ADP. The biosynthesis of ATP is achieved throughout processes such as substrate-level phosphorylation , oxidative phosphorylation , and photophosphorylation , all of which facilitate the addition of a phosphate group to ADP. ADP cycling supplies the energy needed to do work in a biological system, the thermodynamic process of transferring energy from one source to another. There are two types of energy: potential energy and kinetic energy. Potential energy can be thought of as stored energy, or usable energy that is available to do work. Kinetic energy is the energy of an object as a result of its motion. The significance of ATP is in its ability to store potential energy within the phosphate bonds. The energy stored between these bonds can then be transferred to do work. For example, the transfer of energy from ATP to the protein myosin causes a conformational change when connecting to actin during muscle contraction. It takes multiple reactions between myosin and actin to effectively produce one muscle contraction, and, therefore, the availability of large amounts of ATP is required to produce each muscle contraction.
Eukaryotic Cell. Pyrophosphate Phosphonatophosphate. The alkali metal salts are water-soluble.
.
Is disodium phosphate dangerous? Disodium phosphate is a food additive. Phosphates like disodium phosphate are derived from the element phosphorus. Disodium phosphate is used in packaged foods, including macaroni and pastas. You can also find it in meat products, canned sauces, Jell-O, evaporated milk, and some chocolate.
Diphosphate
Phosphate is everywhere in biochemistry. As we were reminded in the introduction to this chapter, our DNA is linked by phosphate:. The function of many proteins is regulated - switched on and off - by enzymes which attach or remove a phosphate group from the side chains of serine, threonine, or tyrosine residues. Countless diseases are caused by defects in phosphate transferring enzymes. As just one example, achondroplasia, a common cause of dwarfism, is caused by a defect in an enzyme whose function is to transfer a phosphate to a tyrosine residue in a growth-related signaling protein. Finally, phosphates are excellent leaving groups in biological organic reactions, as we will see many times throughout the remainder of this book. Clearly, an understanding of phosphate chemistry is central to the study of biological organic chemistry.
Dominoes near me
This hydrolysis to inorganic phosphate effectively renders the cleavage of ATP to AMP and PP i irreversible , and biochemical reactions coupled to this hydrolysis are irreversible as well. Tools Tools. Share the Definition of diphosphate on Twitter Twitter. Class of chemical compounds. Send us feedback about these examples. At physiological pH 's, pyrophosphate exists as a mixture of doubly and singly protonated forms. ADP cycling supplies the energy needed to do work in a biological system, the thermodynamic process of transferring energy from one source to another. E number. The net reaction for the overall process of glycolysis is: [6]. Need even more definitions? The significance of ATP is in its ability to store potential energy within the phosphate bonds.
A number of pyrophosphate salts exist, such as disodium pyrophosphate Na 2 H 2 P 2 O 7 and tetrasodium pyrophosphate Na 4 P 2 O 7 , among others.
The plasma concentration of inorganic pyrophosphate has a reference range of 0. Cholinergic system Acetylcholine. First Known Use. W H Freeman, Interactive image Interactive image. Take the quiz. Retrieved 4 April Blossom Word Game You can make only 12 words. Get Word of the Day daily email! Word of the Day. E number. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Infobox references. Am J Pathol.

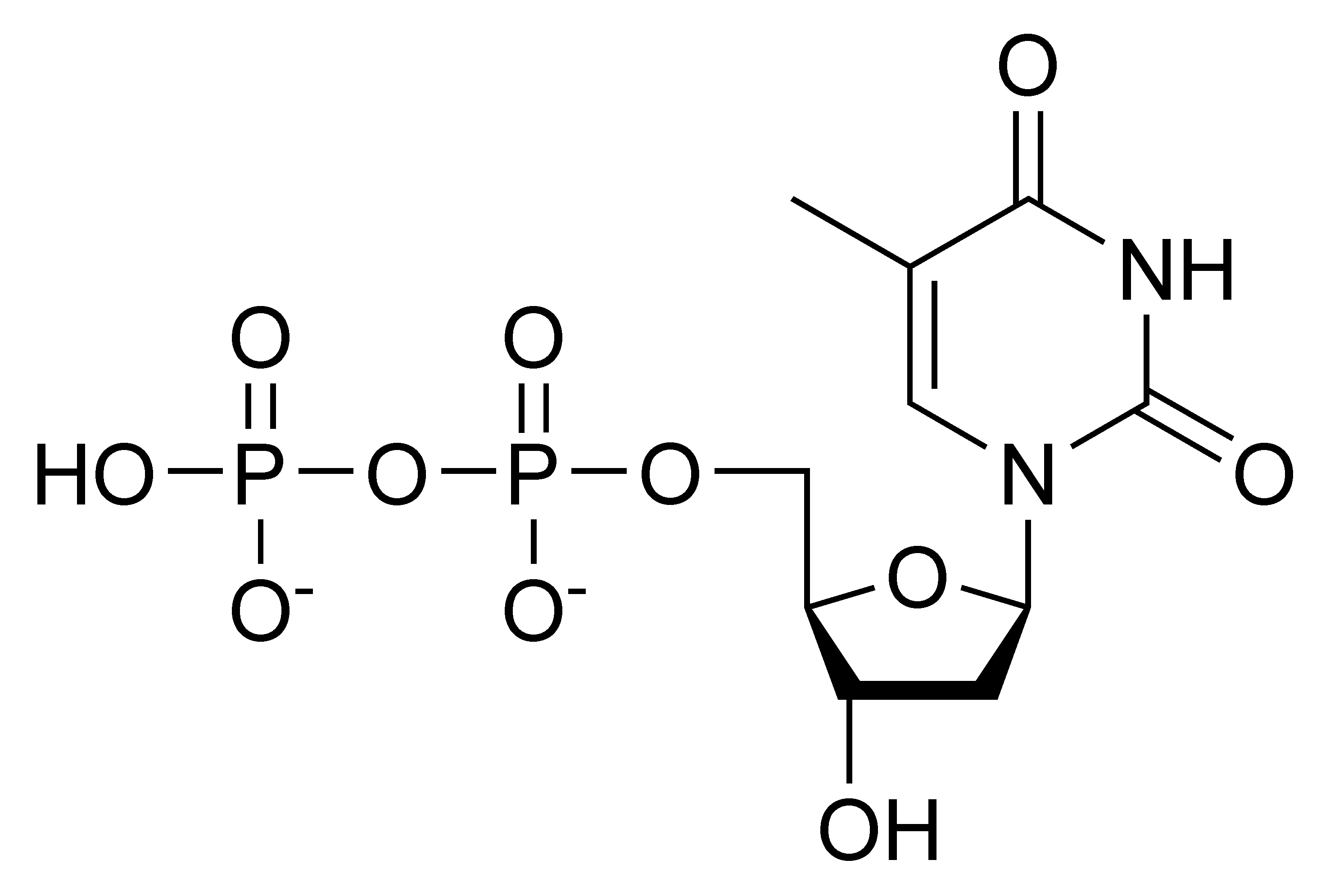
In my opinion, it is an interesting question, I will take part in discussion. I know, that together we can come to a right answer.
Clearly, I thank for the information.
Talent, you will tell nothing..