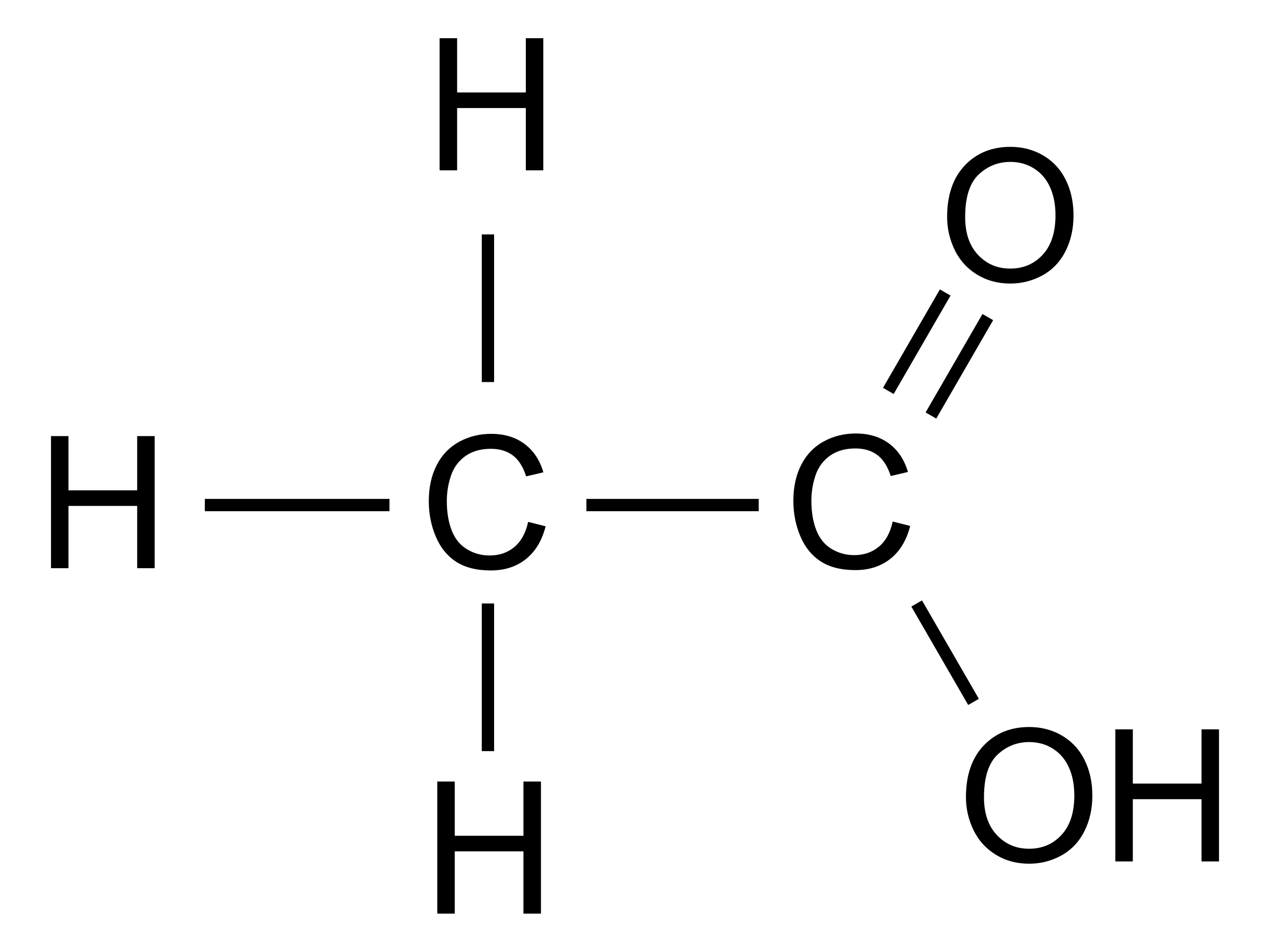
Draw the lewis structure for acetic acid
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a CH3COOH moleculefirst of all you should know the valence electrons present in carbon atomhydrogen atom as well as oxygen atom. Valence electrons are the electrons that draw the lewis structure for acetic acid present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table.
Cheers for this great post. I believe that you have raised some interesting poinst here. Keep up the excellent blog. Created by MakeTheBrainHappy. You could alternatively also draw the structure by including two dots for every bond. As you can see every single element has a filled valence shell with the two oxygen's each containing two lone pairs of electrons, the only instance of this phenomena within the Lewis Structure. In a sense this is a modified structure of methane CH4 with the replacement of one hydrogen with the replacement of a carboxylic group -COOH.
Draw the lewis structure for acetic acid
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Place the remaining six valence electrons around the oxygen atoms in pairs. One oxygen will have two lone pairs and two bonding pairs of electrons in the atom attached to hydrogen. The remaining oxygen bonded to carbon will have three lone pairs and one bonding pair of electrons. Lone pairs are non-bonding pairs of electrons.
So you have seen the above image by now, right?
.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon.
Draw the lewis structure for acetic acid
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons. Carbon C contributes 4 valence electrons, hydrogen H has 1 valence electron, and oxygen O contributes 6 valence electrons each. Calculate the total valence electrons as follows:. Select the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Then connect the first central carbon atom to each of the three hydrogen atoms using single bonds electron pairs. Connect the second central carbon atom to each of the two oxygen atoms and one hydrogen atom, respectively.
Alcoholics anonymous meetings
After shifting this electron pair, the carbon atom will get 2 more electrons and thus its total electrons will become 8. Carbon is group 14 element on the periodic table. The remaining oxygen bonded to carbon will have three lone pairs and one bonding pair of electrons. You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons. Hacker News. The formal charge of an atom should be as close to zero as possible. As mentioned above acetic acid is also present in vinegar which has a variety of household uses; however, the acetic acid is diluted in water to a greater degree than in a research lab. Nearly one third of produced acetic acid is utilized in order to produce "Elmer's glue" material. You could alternatively also draw the structure by including two dots for every bond. Draw the carbon chain, then put the oxygens and finally the hydrogens.
Each oxygen atom has 2 two lone pairs. Also, there are no charges on atoms and acetic acid also does not have an overall charge. Each step is explained in detail in this tutorial.
In a sense this is a modified structure of methane CH4 with the replacement of one hydrogen with the replacement of a carboxylic group -COOH. Read more about our Editorial process. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. Let me explain the above image in short. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen. Draw the carbon chain, then put the oxygens and finally the hydrogens. What is the Lewis structure of acetic acid? He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Due to the importance of different alcohols such as beer and wine in early civilizations, vinegar became one of the earliest chemical substances that was familiar to ancient peoples. Copy short link.

I am sorry, it does not approach me. Who else, what can prompt?