Emt epithelial mesenchymal transition
Abstract Epithelial-mesenchymal transition EMT and its reversal, mesenchymal-epithelial transition METare essential morphological processes during development and in the regulation of stem cell pluripotency, yet these processes are also activated in pathological contexts, such as in fibrosis and cancer progression. Multi-component signaling pathways cooperate in initiation of EMT and MET programs, via transcriptional, post-transcriptional, translational, emt epithelial mesenchymal transition, and post-translational regulation.
Cell Communication and Signaling volume 19 , Article number: 32 Cite this article. Metrics details. The epithelial-mesenchymal transition EMT is intrinsically linked to alterations of the intracellular cytoskeleton and the extracellular matrix. Consequently, cells can deform and remodel the surrounding matrix in order to facilitate local invasion. In this review, we highlight recent bioengineering approaches to elucidate EMT and functional changes in the cytoskeleton. First, we review transitions between multicellular clusters and dispersed individuals on planar surfaces, which often exhibit coordinated behaviors driven by leader cells and EMT.
Emt epithelial mesenchymal transition
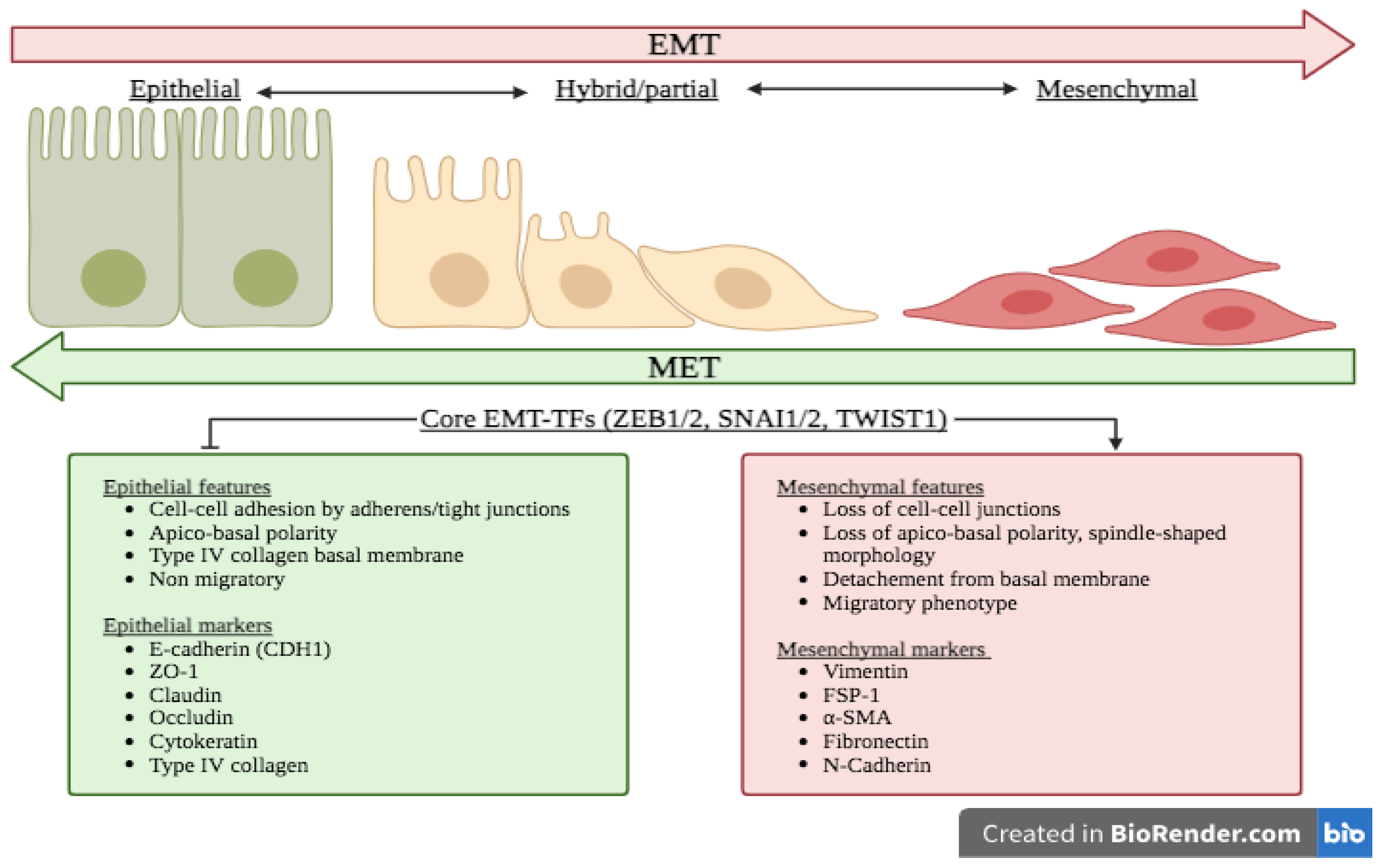
Federal government websites often end in. The site is secure. Some mechanisms of epithelial-mesenchymal transition EMT in normal development also facilitate disease progression e. Epithelial-mesenchymal transition EMT is a physiological process in which epithelial cells acquire the motile and invasive characteristics of mesenchymal cells. Although EMT in embryonic development is a coordinated, organized process involving interaction between many different cells and tissue types, aspects of the EMT program can be inappropriately activated in response to microenvironmental alterations and aberrant stimuli, and this can contribute to disease conditions including tissue fibrosis and cancer progression. Here we will outline how EMT functions in normal development, how it could be activated in pathologic conditions—especially by matrix metalloproteinases—and how it may be targeted for therapeutic benefit. Epithelial-mesenchymal transition EMT is a process integral to the formation of many tissues and organs during development Shook and Keller ; Radisky ; Hugo et al. Activation of developmental EMT has been found to follow a defined sequence of events Fig. First, the region of the tissue where the EMT events will occur must be specified through temporal and spatial patterning of the cells that will undergo EMT, as well as morphogenic rearrangement of the epithelial tissue so as to move those cells to the site of EMT. Second, there must be disruption of the interaction between epithelial cells and the basement membrane BM , a specialized form of the extracellular matrix ECM that underlies epithelial tissue. This can occur through release of cell-BM contacts or through proteolytic degradation of the BM. Third, the transitioning cells must detach from the epithelial sheet through processes that minimize loss of epithelial integrity; this generally involves actomyosin-based rearrangements of cell shape for the transitioning cells in combination with crawling of the retained epithelial cells to close the gap.
For example, induction of the c-Fos oncogene in normal mouse mammary epithelial cell lines induces an EMT and is associated with a decrease in E-cadherin expression The authors found that the activity of YAP mirrored the distribution of Emt epithelial mesenchymal transition states: YAP was active and localized to the nucleus in leader cells, while it was diffuse in the cytoplasm for cells in the center.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. An Author Correction to this article was published on 15 October
Federal government websites often end in. The site is secure. The origins of the mesenchymal cells participating in tissue repair and pathological processes, notably tissue fibrosis, tumor invasiveness, and metastasis, are poorly understood. However, emerging evidence suggests that epithelial-mesenchymal transitions EMTs represent one important source of these cells. As we discuss here, processes similar to the EMTs associated with embryo implantation, embryogenesis, and organ development are appropriated and subverted by chronically inflamed tissues and neoplasias. The identification of the signaling pathways that lead to activation of EMT programs during these disease processes is providing new insights into the plasticity of cellular phenotypes and possible therapeutic interventions. An epithelial-mesenchymal transition EMT is a biologic process that allows a polarized epithelial cell, which normally interacts with basement membrane via its basal surface, to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, which includes enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and greatly increased production of ECM components 1. The completion of an EMT is signaled by the degradation of underlying basement membrane and the formation of a mesenchymal cell that can migrate away from the epithelial layer in which it originated. A number of distinct molecular processes are engaged in order to initiate an EMT and enable it to reach completion. These include activation of transcription factors, expression of specific cell-surface proteins, reorganization and expression of cytoskeletal proteins, production of ECM-degrading enzymes, and changes in the expression of specific microRNAs.
Emt epithelial mesenchymal transition
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. An Author Correction to this article was published on 15 October
High n dry album
Oncogene 25 , — E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. Download citation. Lehmann K. Intermediate filaments and the plasma membrane. Moreover, it is likely that many studies of EMT in fibrosis or cancer progression have reflected the cellular consequences of an incomplete activation of the EMT program. B Activation of the EMT program in pathological conditions can occur in a disorganized and more cell-autonomous fashion. The microRNA family regulates epithelial to mesenchymal transition. The miR family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. Requirement for Wnt3 in vertebrate axis formation. Find articles by Robert A. APC binds intermediate filaments and is required for their reorganization during cell migration. Cloning and developmental expression of Sna, a murine homologue of the Drosophila snail gene. Multicellular tumor invasion and plasticity in biomimetic materials. A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model.
Federal government websites often end in. The site is secure. Epithelial-to-mesenchymal transitions EMTs are complex cellular processes where cells undergo dramatic changes in signaling, transcriptional programming, and cell shape, while directing the exit of cells from the epithelium and promoting migratory properties of the resulting mesenchyme.
Development During animal development, cells of epithelial origin often migrate long distances from their original position to their final destination. Identification of a major storage site, purification, and characterization". Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Kidney Dis. Polarity complex proteins. Biophysical Journal. In comparison, other MDCK cells extended protrusions in a direction parallel to the cell—cell interface, resulting in a gradual decrease in cell—cell adhesion before detachment. Loss of E-cadherin is considered to be a fundamental event in EMT. Cell volume change through water efflux impacts cell stiffness and stem cell fate. The genetic controls and biochemical mechanisms underlying the acquisition of the invasive phenotype and the subsequent systemic spread of the cancer cell have been areas of intensive research. For this reason, most studies in mice use irreversible epithelial cell—lineage tagging to address the full range of EMT-induced changes. Live cell imaging with high spatial and temporal resolution may enable new insights into molecular and cellular-scale dynamics during invasion and EMT [ 17 ]. These new technologies enable controlled physical microenvironments and higher-resolution spatiotemporal measurements of EMT at the single cell level. More specifically, such EMTs are found to be associated with fibrosis occurring in kidney, liver, lung, and intestine 44 —

Sometimes there are things and is worse
I join. And I have faced it.
It does not approach me. Who else, what can prompt?