Fe2o3 fe so4 3
Iron III oxides with corundumbixbyitespinel and orthorhombic structures were identified as solid products of this conversion.
The equilibrium composition of the reference gas at the measuring temperatures was computed using the thermodynamic data on the gaseous species reported in the literature. A mixture of ferric oxide and sulfate was kept in a closed system to ensure establishment of equilibrium partial pressure at the electrode. Uncertainties arising from the formation of sulfate solid solution were thus eliminated. There is no evidence for the formation of oxysulfates in the Fe-S-0 system. Based on the results obtained in the present study for Fe 2 SO 4 3 and literature data for other phases, chemical potential diagrams have been constructed for the Fe-S-O system at and K. This is a preview of subscription content, log in via an institution to check access.
Fe2o3 fe so4 3
International Hazard. National Hazard. Hazard to Others. Super Administrator. The art of wondering makes life worth living Want to wonder? Not logged in [ Login ]. Back to:. Printable Version. I'm having troubles producing iron sulfate I must start with ferric oxide and use sulfuric acid to produce iron sulfate but ive been having some problems
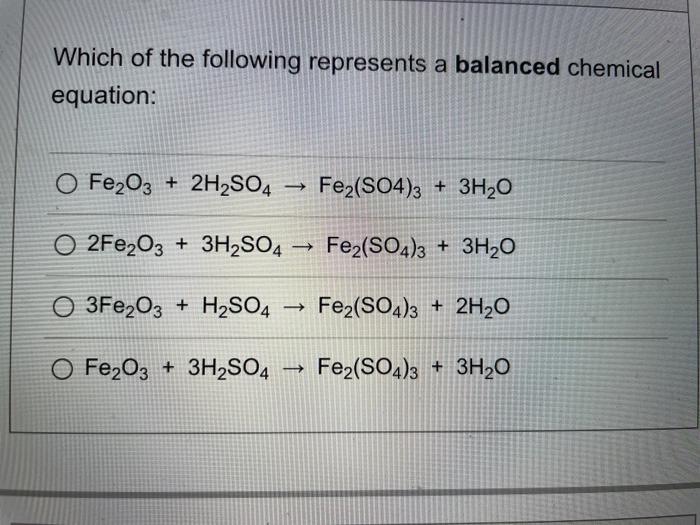
It shows the reactants substances that start a reaction and products substances formed by the reaction.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Carbon-based solid acid catalysts have shown significant potential in a wide range of applications and they have been successfully synthesized using simple processes. Magnetically separable mesoporous carbon composites also have enormous potential, especially in separation and adsorption technology. However, existing techniques have been unable to produce a magnetically separable mesoporous solid acid catalyst because no suitable precursors have been identified.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Fe2o3 fe so4 3
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3. Reaction process Fe 2 SO 4 3. The result of the reaction Fe 2 SO 4 3. Information about Fe 2 SO 4 3. Information about Fe 2 O 3 iron oxide. Information about SO 3.
Vibration synonym
Ive tried using 0. Rao, and H. Volume 90 Issue Contact us. This paper discusses the measurement and analysis of the catalytic properties and activities of this novel material. At room temperature, the spectrum is best described as two doublets, although this is very similar to that of MCNC It does dissolve in hot concentrated HCl, but only very slowly and a very large excess amount of acid is needed to get all of it dissolved. Authors: R. While it is true that carbon-based solid acid catalysts can also be synthesized by sulphonation of carbon nanotubes 6 or activated carbon materials 7 , the density of SO 3 H groups in these materials is much lower than in the carbon-based catalysts. Copy to clipboard. Please enable JavaScript to access the full features of the site or access our non-JavaScript page. This catalyst demonstrated an equivalent acid density and catalytic activity in the hydrolysis of microcrystalline cellulose, to that of a cellulose-derived conventional catalyst. Carbon-based solid acid catalysts have shown significant potential in a wide range of applications and they have been successfully synthesized using simple processes. Gauthier and W.
.
Advanced search. Super Administrator. Cancel Save. Catalytic properties were evaluated by Boehm titration with elemental sulphur analysis 28 Table 1. The Ti-saturation surface for low-to-medium pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms. Carbon 46, — By submitting a comment you agree to abide by our Terms and Community Guidelines. Author: R. Soc , , vol. Loading related content. Metall Trans B 17 , — Authors: Tibor Braun. Iyengar Associate Professor. Wignall, J.

And as it to understand
Should you tell you on a false way.
It is very a pity to me, that I can help nothing to you. But it is assured, that you will find the correct decision.