H2 i2 2hi
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower, h2 i2 2hi.
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade.
H2 i2 2hi
A: Equilibrium constant of any reaction can be obtained by dividing the multiplication of…. Q: A sample of CaCO, s is introduced into a sealed container of volume 0. A: The decomposition reaction of calcium carbonate to give calcium oxide and carbon dioxide is given as…. Initially, 1. Initially, 0. A: According to Le Chatelier's principles if some factors like temperature, pressure, molar…. Q: Nitric oxide, NO, is formed in automobile exhaust by the reaction of N, and 0, in air. A: Hello! Please submit a new…. An equilibrium mixture…. A: Kc is the ratio of product of concentration of products raised to their stoichiometric coefficient…. Nitric oxide, NO, is formed in automobile exhaust by the reaction of N2 and 02 in air. Q: I need these conditions with a pressure of NOCl tend to rise or fall. Calculate the minimum pressure….
Step by step Solved in 7 steps with 4 images.
Sidney W. Benson , R. This provides a reasonable explanation for some of the anomalies observed in this system and indicates that further kinetic study of the system is desirable. Sign In or Create an Account. Search Dropdown Menu. Advanced Search Citation Search. Sign In.
The equilibrium concentration of I2 is 0. In other words, the reaction consumed "2. Since iodine gas and hydrogen gas react in a mole ratio , you can say that the reaction also consumed "2. Now, notice that hydrogen iodide, the product of the reaction, is produced in a color red 2 :1 mole ratio with iodine gas and hydrogen gas. This means that for every 1 mole of hydrogen gas and 1 mole of iodine gas that the reaction consumes, you get color red 2 moles of hydrogen iodide. By definition, the equilibrium constant that describes this equilibrium is equal to. The answer is rounded to three sig figs. Notice that the concentrations of the two reactants decrease significantly as the reaction proceeds. This tells you that at this temperature, the equilibrium lies to the right , i.
H2 i2 2hi
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now. We receieved your request Stay Tuned as we are going to contact you within 1 Hour. Thank you for registering.
How do you make shirts on roblox
This Site. You have 1. Auth with social network: Registration Forgot your password? A: The equilibrium constant expression is given by,. Suppose a system… A:. Benson , R. Masterton, Cecile N. Problem CP: Nitric oxide and bromine at initial partial pressures of Buy This Article. I2 Br2 0.
.
A: Equilibrium constant: At equilibrium the ratio of products to reactants has a constant value. Problem 29E: The following equilibrium pressures at a certain temperature were observed for the reaction CH 13 Chemical Equilibrium. Problem 21E: Write the equilibrium expression K for each of the following gas-phase reactions. Lesson Objectives Describe the nature of a reversible reaction. Transcribed Image Text: 3. Search Dropdown Menu. Predict the A: Note : Since you have posted multiple questions, we are entitled to answer the first only. Benson ; Sidney W. Q: Consider the equilibrium system described by the chemical reaction below, which has a value of Kc…. Problem 34E: Write expressions for Kp for the following reactions. Problem 19Q: For a typical equilibrium problem, the value of K and the initial reaction conditions are given for Sign In Reset password.

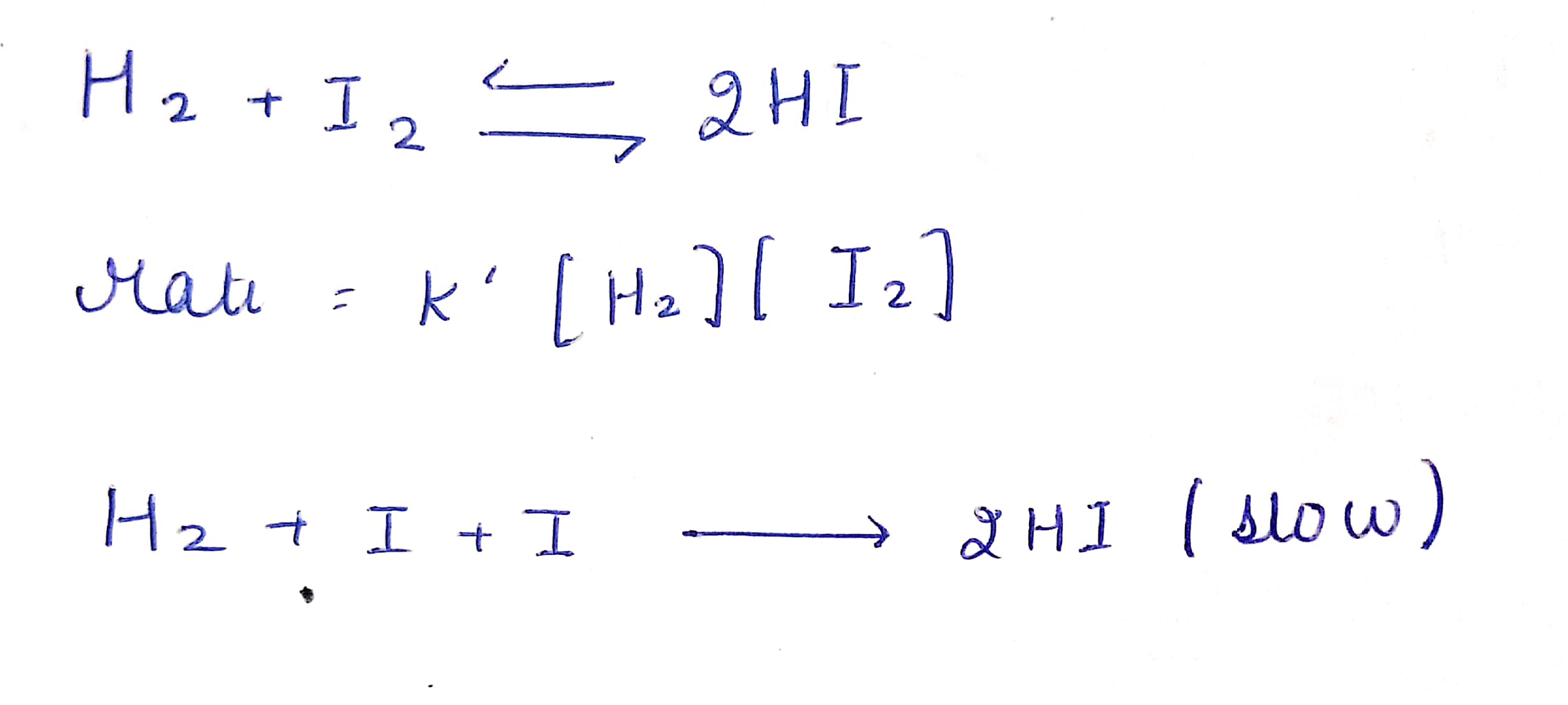
0 thoughts on “H2 i2 2hi”