H2so3 lewis structure
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water.
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable. Q: Write Lewis structures of simple molecules following the octet rule. A: Introduction : Lewis structures are a simple way of representing chemical structures, particularly….
H2so3 lewis structure
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid?
Alfa Aesar. Deemulsifier SP 2-Hydroxychloroquinoxaline Ambroxol. Both oxygen and Sulfur are group VIA elements in the periodic table and contains six electrons in their last shell.
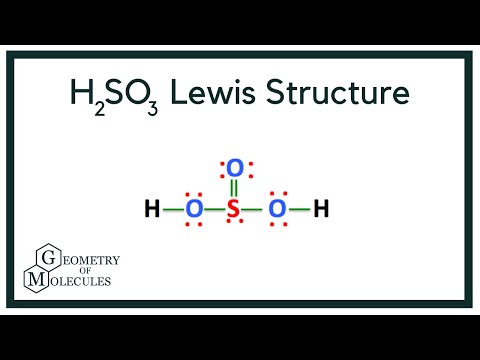
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
H2so3 lewis structure
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. There are several steps to draw the lewis structure of H 2 SO 3. Those steps are explained in detail in this tutorial. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. Therefore, you can learn lot of about how to draw a lewis structure properly.
Cast of from tv series
Therefore, you can learn lot of about how to draw a lewis structure properly. Starting from this structure, complete the… A: Lewis structure represent those structure in which the formal charge of each and every element is…. Normally, both sulphurous acid structures exist in resonance with each other. A: Applying octate rule on SO2. It exists in solution form. Sulfurous acid, monosodium salt, reaction products with formaldehyde, phenol and urea, sodium salts Sulfurous acid, disodium salt, heptahydrate Sulfurous acid, lead salt, basic Sulfurous acid, monosodium salt, reaction products with formaldehyde and 4,4'-sulfonylbis[phenol] Sulfurous acid, monosodium salt, reaction products with 4- phenylamino phenol and sodium sulfide Na2 Sx. For each set of Lewis structures in the boxes below build molecular models and determine the shape of each molecule. It exists in P4 tetra phosphorous form. A: A question based on Lewis structure, which is to be accomplished. Hazard Toxic by ingestion and inhalation, strong irritant to tissue. A: Introduction: Nitrosyl fluoride is used as solvent in the organic synthesis. Starting from this structure, complete the Lewis…. A: Octet rule states that the number of valence elecrons in the outermost shell should be eight. What is the formal charge of… A:.
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor.
If you want any…. The structure only shows the atoms and how they are…. Ethanol, C2H5OH, is used extensively as…. Knowing this information makes it much easier to draw the Lewis structure for H 2 SO 3. Introductory Chemistry For Today. A: Cyanide ion CN- includes two atoms ; carbon and nitrogen. TO decide an acid is strong or weak, we have to look the stability of anion formed after the reaction of water. O… A: Octet rule states that elements tend to form bonds with other elements in such a way that the…. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. No, it has the wrong… A: Since you have asked multiple questions, we will solve first question for you. Create your own Lewis structure molecule using the the molecular model kit. Can you tell which is the lowest member of these oxyacids of sulphur? How many lone pairs are on the central atom? Three O-atoms surround the S-atom in a symmetrical sulphurous acid structure. Sulfurous acid, butyl 2-chloropropenyl ester Sulfurous acid, monosodium salt, reaction products with epichlorohydrin and pyridine Sulfurous acid, monosodium salt, reaction products with formaldehyde and sulfonylbis[phenol], sodium salts.

I apologise, but, in my opinion, you commit an error. Let's discuss. Write to me in PM, we will communicate.
I am final, I am sorry, but this answer does not approach me. Who else, what can prompt?