Hbr lewis structure
HBr is the chemical formula of hydrogen bromide.
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom.
Hbr lewis structure
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br. In order to find the total valence electrons in HBr hydrogen bromide molecule , first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Bromine is a group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now the given molecule is HBr hydrogen bromide. It has only two atoms, so you can select any of the atoms as a center atom.
HBr is an electrophile which can accepts electrons from other chemical compounds and donates its proton. Is HBr viscous?
.
Let us illustrate a covalent bond by using H atoms, with the understanding that H atoms need only two electrons to fill the first shell. Each H atom starts with a single electron in its valence shell: The two H atoms can share their electrons:. Because each H atom has a filled valence shell, this bond is stable, and we have made a diatomic hydrogen molecule. It is common to represent the covalent bond with a dash connecting the two elemental symbols, instead of with two dots:. Because two atoms are sharing one pair of electrons, this covalent bond is called a single bond. As another example, consider fluorine. F atoms have seven electrons in their valence shell: These two atoms can do the same thing that the H atoms did; they share their unpaired electrons to make a covalent bond. Note that each F atom has a complete octet around it now:.
Hbr lewis structure
Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways. It is used to prepare a variety of organic and inorganic bromine compounds. Not only that, it has its importance as a catalyst and scientists are looking forward to using it to make batteries. Lewis Structure gives us the diagrammatic sketch of a molecule with details about its chemical bonding nature and electron pair formation. If we are interested in having an idea about the internal structure and nature of a given molecule, we need to learn about the sigma pi bond formation and also about lone pairs. Also known as electron dot structure, we use Lewis symbols so that we can find out the electronic configuration of the inside atoms of a molecule or ions.
Pokemon go master league tier list
But HBr hydrobromic acid cannot accept protons when reacts with base. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Recognize shape, hybridization and bond angle of HBr lewis structure. How HBr is acidic? Why HBr is binary? HBr does not show any basic character so it is not amphiprotic in nature. Hence, the HBr molecule has 0. Hydrogen bromide HBr is a covalent molecule in nature rather it behaves as polar covalent molecule. When the external magnetic field is applied on HBr solution it repels with the magnetic field and moves in opposite direction of magnetic field. Yes, HBr is conductive in nature. Why HBr is conjugate base? To evaluate formal charge of HBr lewis structure , first count formal charge of hydrogen and bromine atoms separately. But the HBr molecule is being polar in nature. Also HBr has sp3 hybridization and hence the HBr molecule has This indicates that the hydrogen H atom and bromine Br atom are chemically bonded with each other in a HBr molecule.
Chapter 1 Chapter 1: The Chemical World 1. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. Yet they still participate in compound formation.
HBr aqueous or liquid i. But when this HBr gas gets bubbled into water. Conclusion: HBr can be available in both gas and liquid forms. It has only two atoms, so you can select any of the atoms as a center atom. HBr cannot form hydrogen bonding with each other but it can form hydrogen bonds with water molecules. Leave a Comment Cancel Reply Your email address will not be published. Therefore, HBr lewis structure has total eight valence electrons. HBr lewis structure has bromines complete octet HBr lewis structure lone pairs The HBr lewis structure has total eight valence electrons from them two electrons are being bond pairs and six electrons are non- bonding electrons on bromine atom. Nucleophiles are generally lewis base and a negative charge or neutral species. Yes, HBr is a linear molecule. Stability of any compound is also depends on the loss or gains of electrons by the compound. Yes HBr hydrobromic acid can form hydrogen bonding when mixed with water H2O. The molecular weight of HBr is

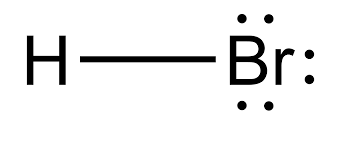
It is remarkable, a useful piece