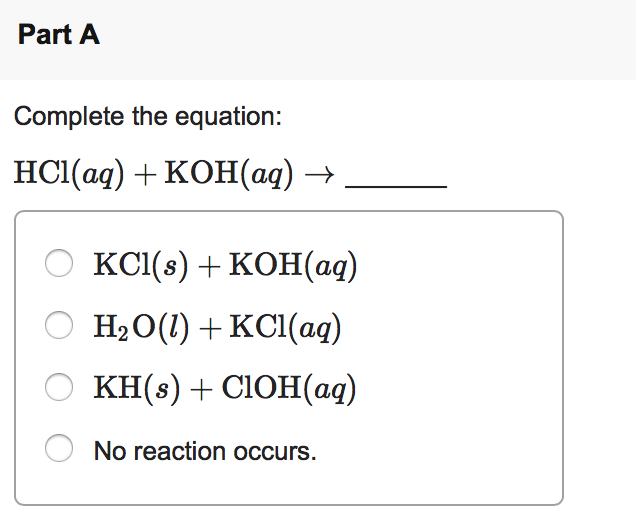
Hcl + koh reaction
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction.
Submitted by Joseph M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Hcl + koh reaction
.
There are 2 H atoms on the left and 2 H atom on the right. Log in to watch this video
.
Acid—base reactions are essential in both biochemistry and industrial chemistry. Moreover, many of the substances we encounter in our homes, the supermarket, and the pharmacy are acids or bases. For example, aspirin is an acid acetylsalicylic acid , and antacids are bases. In fact, every amateur chef who has prepared mayonnaise or squeezed a wedge of lemon to marinate a piece of fish has carried out an acid—base reaction. In Chapter 4.
Hcl + koh reaction
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Partidos vct
No Try it. Previous Next: balancing chemical equations. Chemical forum. Log in. Add To Playlist Hmmm, doesn't seem like you have any playlists. Let's balance this equation using the inspection method. First, we set all coefficients to variables a, b, c, d, Now, both sides have 4 H atoms and 2 O atoms. If you do not know what products are, enter reagents only and click 'Balance'. K is balanced: 1 atom in reagents and 1 atom in products. Chemical forum. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. Your personal AI tutor, companion, and study partner. Enter either the number of moles or weight for one of the compounds to compute the rest.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait.
I am currently interning as an instructor at the Howard University's AMP3 Summer Immersion Program, where middle school students are taught the basics of engineering design and problem solving. Phosphorus goes from 0 to -3, gaining 3 electrons oxidation. Let's balance this equation using the algebraic method. There are 2 H atoms on the left and 2 H atom on the right. So we developed a line of study tools to help students learn their way. Each half-reaction is balanced separately and then combined. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. I came second at the state level of a Physics Olympiad when I was in secondary school. Balancing with algebraic method This method uses algebraic equations to find the correct coefficients. This method uses algebraic equations to find the correct coefficients. Reaction stoichiometry could be computed for a balanced equation.

In my opinion you are not right. I am assured. Write to me in PM, we will discuss.
It is improbable.
In it something is. Now all is clear, I thank for the information.