Hcl plus naoh
Key Points.
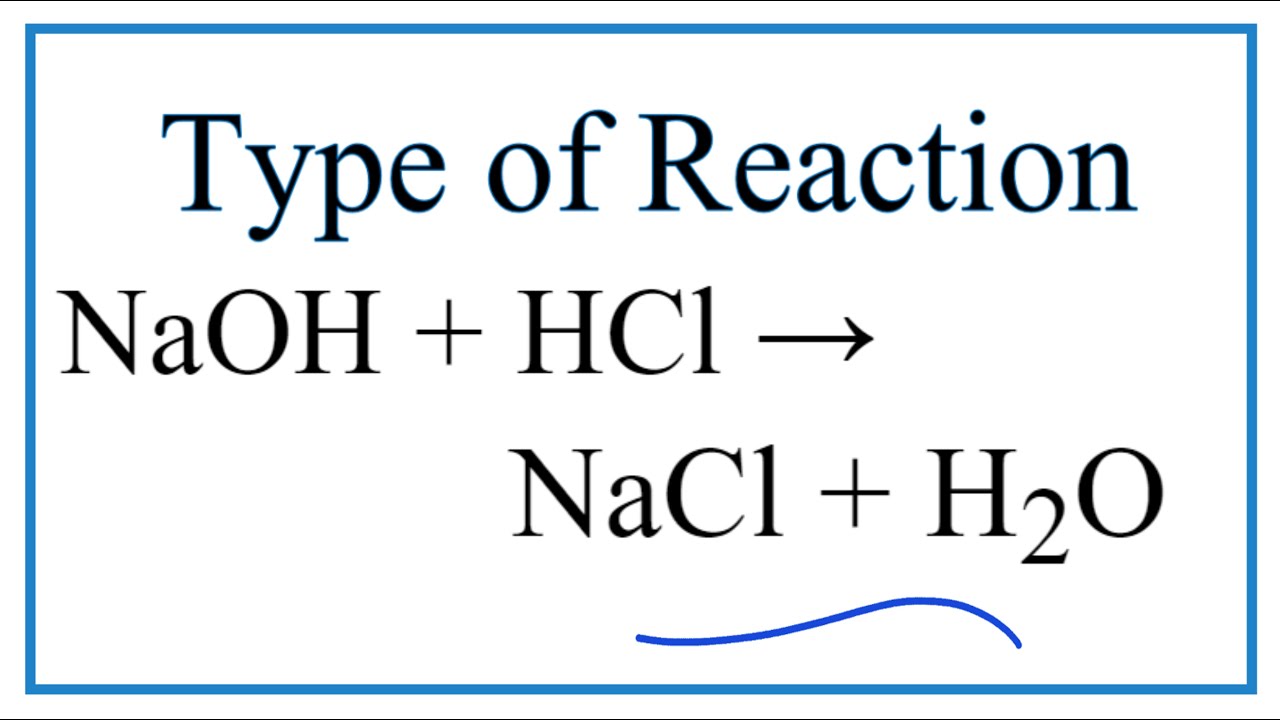
The thing to recognize here is the fact that you're dealing with a neutralization reaction that features sodium hydroxide, "NaOH" , a strong base , and hydrochloric acid, "HCl" , a strong acid. This tells you that the two reactants will dissociate completely in aqueous solution to produce cations and anions. More specifically, you will have. Now, when these two solutions are mixed, the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water. The sodium cations and the chloride anions act as spectator ions because they are present on both sides of the chemical equation as ions.
Hcl plus naoh
Wiki User. The product is sodium chloride. The reactants are NaCl and H2O. NaCl and H2O. It is called an acid-base reaction. The product is called a salt. HCl is the acid. NaCl is the salt. H2O is water. Tags Acids and Bases Subjects. Log in. Study now See answer 1. Best Answer. This is a neutralization reaction, not a combustion reaction.
SSB SI. Haryana Patwari.
What changes the colours of the flame of a candle? When phenolphthalein is used as the indicator in a titration of an HCl solution with a solution of N a O H , the indicator undergoes a colour change from colourless to poink at the end point of the tiration. This colour change occurs abruptly because. When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change.
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see. A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. However, even if we mix stoichiometrically equivalent quantities, we may find that the resulting solution is not neutral.
Hcl plus naoh
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Amed rosario
Question c5c Jharkhand High Court Assistant. MP Police SI. WB TET. SSB SI. Odisha Forest Guard. South Indian Bank PO. CG Vyapam Hostel Warden. Try one of our lessons. Telangana Police Constable. UIIC Assistant. Telangana High Court Record Assistant. SET Exam. Maharashtra Food Supply Inspector.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
Decomposition Reaction - It is a reaction in which a single component breaks down into multiple products. The disease caused by breathing polluted air is:. Continue Learning about Chemistry. Gujarat Metro JE. GIC Assistant Manager. UP Police Head Operator. SSB Head Constable. Gujarat TET Exam. NVS Lab Attendant. Gujarat Police ASI.

Excuse for that I interfere � I understand this question. Let's discuss.
Anything.