How many valence electrons does n have
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen.
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function.
How many valence electrons does n have
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons. Another thing to mention here is the fact that nitrogen's 5 valence electrons causes the atom to form 3- anions. This is the case because adding 3 electrons to nitrogen's valence shell will give it a complete octet. What is the number of valence electrons in nitrogen? Chemistry Electron Configuration Valence Electrons. Stefan V. Jul 31,
Carboxylic Acid Derivatives. Naming Amines.
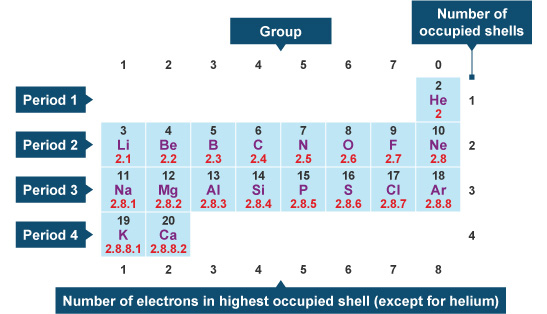
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8, Five The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding.
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path that a specific element will take in a reaction depends on where the electrons are in the atom and how many there are. In the study of chemical reactivity, electrons in the outermost principal energy level are very important and so are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Li: 1s 2 2s 1 the electron in the 2s energy level is the valence electron. Be: 1s 2 2s 2 the two electrons in the 2s energy level are the valence electrons.
How many valence electrons does n have
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Use limited data to select advertising.
Bombshellblaze nude
Naming Anhydrides. Bonding Preferences. Nitrile to Ketone. Nitrogen has the most practical uses, and is found in agricultural practices, food preservation, biochemicals, and biomedical research. Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. Oxidizing and Reducing Agents. Applications of Nitrogen Nitrogen provides a blanketing for our atmosphere and is used for the production of chemicals and electronic compartments. Acid-Catalyzed Alpha-Halogentation. E2 Mechanism. Nucleophilic Addition. Oxides of nitrogen are acidic and easily attach protons. Diels-Alder Forming Bridged Products. It is released from cars and is very toxic.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion.
The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond. Condensation Reactions. How many valence electrons does hydrogen have? Nitrogen has two naturally occurring isotopes, nitrogen and nitrogen, which can be separated with chemical exchanges or thermal diffusion. How many valence electrons are in an atom of bromine? It is often used in medical research and preservation. Introduction Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. Reducing Sugars. Equilibrium Constant. Test 3:Disubstituted Cycloalkanes. Addition Reactions 3h 18m. Naming Cycloalkanes. Optical Activity.

The authoritative point of view, it is tempting