How many valence electrons in o
Oxygen has 6 valence electrons.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Atomic structure and electron configuration. About About this video Transcript.
How many valence electrons in o
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons. How many valence electrons does O have? Oct 30, Explanation: This means that it's outer energy level contains six valence electrons. Related questions How do valence electrons affect chemical bonding?
BPSC Assistant. Generally speaking, if you're talking about elements that are in the S block or the P block, you can think about how many valence electrons they have just based on what column they're in. RRB Staff Nurse.
Key Points. Additional Information. Last updated on Feb 7, Along with the official notification, the apply online link will also be opened. Candidates will be able to apply online until 10th July, This year, SSC completely changed the exam pattern.
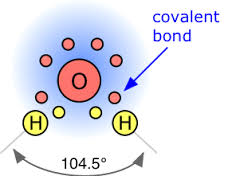
The standard atomic mass of oxygen is Oxygen participates in the formation of bonds through valence electrons. Oxygen is a non-metallic element. Oxygen is an element of group The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell after the electron configuration of oxygen is called the valence electrons of oxygen. The valence electrons determine the properties of the element and participate in the formation of bonds. The electron configuration of oxygen shows that the last shell of oxygen has a total of six electrons. The valence electrons have to be determined by following a few steps. The electron configuration is one of them.
How many valence electrons in o
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons.
Vmath
CG Vyapam Assistant Grade 3. WB TET. ISRO Draughtsman. BRO Vehicle Mechanic. Indian Coast Guard. So And so you'd say, alright, well maybe they can grab those electrons from something else and that's actually what oxygen does a lot of, it grabs electrons from other things. Which of the following element will become stable after losing an electron? Cod liver oil obtained from fish is rich in:. Rajasthan High Court Civil Judge. Assam Police Jail Warder. BPSC Assistant. Bihar LRC Clerk. MP Vyapam Sub Engineer. Army Havildar SAC.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections.
Oil India Grade 3. Kerala Beat Forest Officer. AP Police SI. NFC Stipendiary Trainee. But fluorine has 7 valence electrons, 1 away from being filled. NVS Staff Nurse. Uttarakhand Police SI. Civil Services Exam. Learn today! Telangana High Court Office Subordinate. Once you reach the fourth period and the transition metals they follow an 18 electron rule of stability, but it's the same idea as before in that they are attempting to fill their valence electron shells in order to become stable. The homolytic fission of a covalent bond liberates :. Why does my textbook have, for instance, have the elctron config of phosphorus as 1s2 2s2 2p6 3s2 3px 1 3py1 3pz1 What are the x's y's and z's for? AP TET. MP Patwari.

Thanks, has left to read.
I congratulate, what necessary words..., an excellent idea