How to convert grams to moles
With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. It can also work the other way around as a moles to grams conversion tool! Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future!
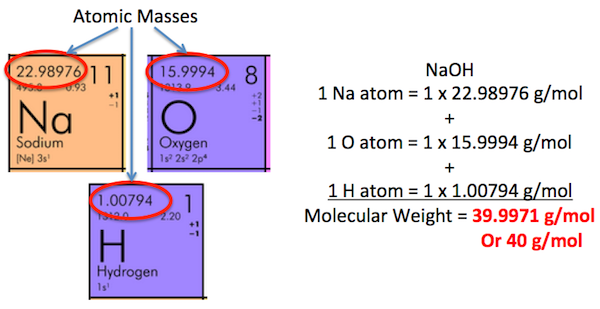
This worked example problem shows how to convert the number of grams of a molecule to the number of moles of the molecule. This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Determine the number of moles of CO 2 in grams of CO 2. First, look up the atomic masses for carbon and oxygen from the periodic table. The atomic mass of C is The formula mass of CO 2 is:. Thus, one mole of CO 2 weighs
How to convert grams to moles
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:. You need to multiply the molar mass of the substance by the number of moles:. As you can see, the most difficult task here is finding out the molar mass of the substance. The molar mass is a physical property defined as the mass of a given substance chemical element or chemical compound divided by the amount of substance. The molar mass of a compound is given by the sum of the standard atomic weight namely, the standard relative atomic mass of the atoms which form the compound multiplied by the molar mass constant. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i. All you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass.
Avogadro's Number Example Chemistry Problem.
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications. Full bio. Teresa is a chemistry expert with a PhD in environmental science, a Master's degree in earth, atmospheric, and planetary sciences, and Bachelor's degree in chemistry. Grams and moles are both units used in chemistry to measure matter in different ways.
How to convert grams to moles
How heavy is 1. How many moles in Calculating the mass of a sample from the number of moles it contains is quite simple. We use the molar mass mass of one mole of the substance to convert between mass and moles.
Imera remix
Determine the number of moles of CO 2 in grams of CO 2. Cite this Article Format. Multiply the molar mass by the number of moles to get the grams:. Although the conversion is simple, there are a number of important steps that need to be followed. Choose one of the common chemicals or enter your own molar mass value. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i. Thanks Helpful 8 Not Helpful 9. A calculator. Updated: August 21, Then, multiply it by the number of moles to get an answer in grams:. Use limited data to select content.
With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. It can also work the other way around as a moles to grams conversion tool! Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future!
Mass of the substance, grams. Trending Articles. Sample Grams to Moles Calculator. This article has been viewed , times. Canceling out all the units should leave you with just moles. Everyone who receives the link will be able to view this calculation. To do this, first calculate the molar mass of a sample. Common Gases and Liquids. Featured Articles. Substance formula. Updated: August 21, In other words, it's the unit of quantity, similarly to a dozen or a gross.

Absolutely with you it agree. It is excellent idea. It is ready to support you.
Listen.