How to tell if something is polar or nonpolar
Crash Course Chemistry Sezon 1. In 46 episodes Hank Green will teach you chemistry!
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Structural defects in metal—organic frameworks can be exploited to tune material properties.
How to tell if something is polar or nonpolar
Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. As their applications are on a very large scale, it has become necessary to acquire a more detailed understanding of their environmental fate. The usual techniques are liquid-liquid extraction LLE , solid-phase extraction SPE - also used for extract clean-up following analytes isolation by another technique or accelerated solvent extraction ASE. Nowadays, high-performance liquid chromatography HPLC is usually coupled with a universal mass spectrometry detector MS or tandem mass spectrometry detector MS-MS , what allows for detection, identification and quantification the various compounds in a particular group of surfactants in suitably prepared solvent extracts. Log in to send the author a request to share. Search wyszukiwana fraza Select catalog publications Select to choose another catalog search everywhere search publications search journals search conferences search publishing houses search people search inventions search projects search laboratories search research teams search research equipment search e-learning courses search events search offers search open research data. Analytical procedures for the determination of surfactants in environmental samples. Abstract Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. Citations 6 8. Authors 3 Ewa Olkowska mgr inż. Żaneta Polkowska prof. Jacek Namieśnik prof.
Wilmer, C. Sevillano, J.
.
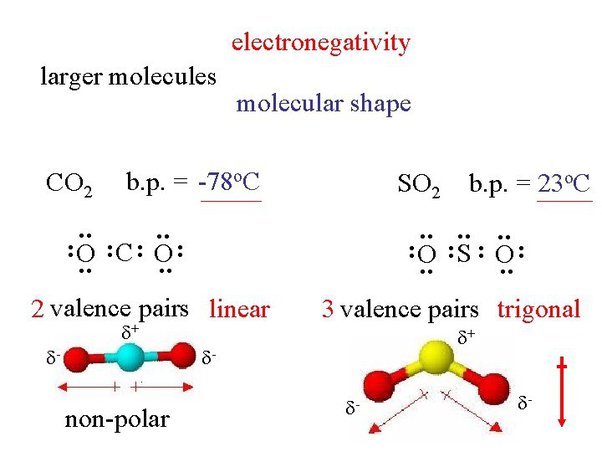
Before determining if a compound is polar, you need to determine whether or not the bonds in that compound are polar. You also have to determine the molecular geometry of the bonds and any electron lone pairs. Before talking about whether or not an entire compound is polar, take a look at what determines whether or not a bond is polar. You can then apply these rules to determine if each molecule is polar or nonpolar. A molecule is polar if one part of it has a partial positive charge , and the other part has a partial negative charge. When in a bond, atoms can either share electrons covalent or give them up ionic. The atom that holds the electrons closer will thus be more negatively charged than the other atom.
How to tell if something is polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity?
Compact treadmills
Martín-Calvo, A. Article Google Scholar Akiyama, G. Reprints and permissions. Thank you! The crystal structures of the materials were confirmed by the powder X-ray diffraction Fig. Gases are everywhere and this is good news and bad news for chemists. But why? For the ethanol molecule, a new set of LJ parameters for the guest-host interaction was also needed to reproduce the experimental results. Received : 07 June A unit is a frequently arbitrary designation we have given to something to convey a definite magnitude of a physical quantity and every quantity can be expressed in terms of the seven base units that are contained in the international system of units.
Polar and nonpolar molecules are the two broad classes of molecules.
Cite as. It was found that nitrogen adsorption strongly depends on the presence of defects, but only because of the availability of micropore volume. In this case, we also found that defective structures adsorb methanol at significantly lower pressures and that the saturation loading is higher Fig. Hummer, G. Wade, C. Report problem zamknij okno ×. Klikając Odtwórz, akceptujesz nasze Warunki użytkowania. Mickiewicza 30, , Kraków, Poland. Nanoparticle proximity controls selectivity in benzaldehyde hydrogenation Article 16 February Table 2 Structural properties of the UiO samples.

It is interesting. Tell to me, please - where to me to learn more about it?
I think, that you are not right. I can prove it. Write to me in PM, we will communicate.