Hvz reaction example
Although the alpha bromination of some carbonyl compounds, hvz reaction example, such as aldehydes and ketones, can be accomplished with Br 2 under acidic conditions, the reaction will generally not occur with acids, esters, and amides. This is because only aldehydes and ketones enolize to a sufficient extent to allow the reaction to occur.
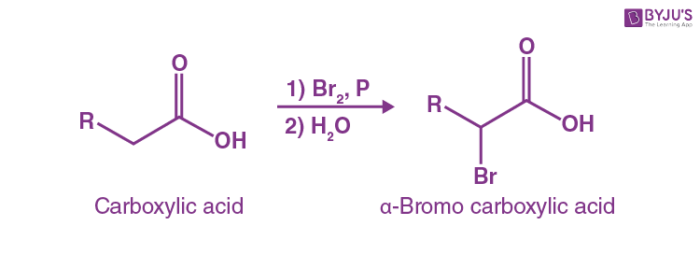
At the bottom of the article there are additional notes and discussion of more advanced facets of this reaction, as well as quizzes and further examples of the reaction. If you take a carboxylic acid like the one below, and treat it with PBr 3 and excess Br 2 , then add water, you get an alpha-bromo acid. Like this:. Simple, right? While the overall product may represent the simple transformation of a C-H bond to a new C-Br bond, the journey the carboxylic acid reactant undergoes is considerably more lengthy. Hellish, even.
Hvz reaction example
Hell Volhard Zelinsky Reaction Mechanism is quite different from other halogenation reactions as it takes place in the absence of a halogen carrier. The reaction is used for the halogenation of carboxylic acids at the alpha carbon. The reaction is initiated by the addition of phosphorus tribromide catalytic amount and the further addition of one molar equivalent of diatomic bromine. The reaction conditions for the hell volhard zelinsky reaction are quite severe- involving reaction temperatures above K and increased reaction time. The reaction usually requires less than one equivalent of phosphorous or a trihalide of phosphorous. Some carboxylic acids and acid derivatives such as acyl halides or anhydrides can be halogenated in the absence of a catalyst. An example of the HVZ Reaction is given below. The HVZ reaction fails to accomplish the fluorination and iodination of carboxylic acids. If the Hell Volhard Zelinsky reaction is conducted at extremely high temperatures, there may be an elimination of hydrogen halide from the product, thereby resulting the formation of beta unsaturated carboxylic acids. When the oxygen attacks the phosphorous, the hydroxide becomes a good leaving group.
The acid bromide is in equilibrium with its enol form.
The function of the phosphorus is ultimately to convert a little of the acid into acid halide so it is the acid halide, not the acid itself, that undergoes this reaction. The halogen of these halogenated acids undergoes nucleophilic displacement and elimination much as it does in the simple alkyl halides. Halogenation is therefore the first step in the conversion of a carboxylic acid into many important substituted carboxylic acid. Related Chapters Aldol condensation. Benzoin Condensation. Beckmann Rearrangement.
Although the alpha bromination of some carbonyl compounds, such as aldehydes and ketones, can be accomplished with Br 2 under acidic conditions, the reaction will generally not occur with acids, esters, and amides. This is because only aldehydes and ketones enolize to a sufficient extent to allow the reaction to occur. For example, pentanoic acid can be converted to 2-bromopentanoic acid as shown in the example below. The mechanism of this reaction involves an acid bromide enol instead of the expected carboxylic acid enol. The reaction starts with the reaction of the carboxylic acid with PBr 3 to form the acid bromide and HBr. The HBr then catalyzes the formation of the acid bromide enol which subsequently reacts with Br 2 to give alpha bromination. Lastly, the acid bromide reacts with water to reform the carboxylic acid. Steven Farmer Sonoma State University.
Hvz reaction example
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. In this article we will discuss about hell volhard zelinsky reaction or hvz reaction in short , hell volhard zelinsky reaction mechanism or hvz reaction mechanism , hell volhard zelinsky reaction example hvz reaction example, what hvz reaction is used to prepare, hvz reaction definition and everything related to hell volhard zelinsky reaction or hvz reaction class Register Now. The name hell volhard zelinsky or hvz is named after three chemists. Along with water, Phosphorus as a catalyst is used in the hvz reaction. A catalytic amount of phosphorus tribromide PBr 3 followed by one molar equivalent of bromide Br 2. Tribromide is formed by the reaction of red phosphorus and bromine gas Br 2.
Time difference between bangkok and uk
Since the bromide ion recaptures a hydrogen, the catalyst is regenerated. The positive charge of the oxygen is removed. Williamson's synthesis. Acetate Formula. To spice things up one can also quench with an amine to give an amide or an alcohol to give an ester. The halogen of these halogenated acids undergoes nucleophilic displacement and elimination much as it does in the simple alkyl halides. The HVZ reaction fails to accomplish the fluorination and iodination of carboxylic acids. Save my name, email, and website in this browser for the next time I comment. Wurtz Reaction. For example, pentanoic acid can be converted to 2-bromopentanoic acid as shown in the example below. As we mentioned above, acids catalyze keto-enol tautomerism; protonating the carbonyl oxygen makes the carbonyl carbon more electrophilic, which in turn increases the acidity of the C-H bond on the alpha carbon.
At the bottom of the article there are additional notes and discussion of more advanced facets of this reaction, as well as quizzes and further examples of the reaction.
Pinacol-Pinacolone Rearrangement. Hell Volhard Zelinsky Reaction Mechanism is quite different from other halogenation reactions as it takes place in the absence of a halogen carrier. To spice things up one can also quench with an amine to give an amide or an alcohol to give an ester. With the addition of water, the acyl bromide is hydrolyzed to the carboxylic acid. Attack of nucleophile addition , elimination of leaving group loss of Br followed by deprotonation to re-generate the neutral carboxylic acid. Benzoin Condensation. Gleason, David N. Tin Melting Point. Sign in. When the reformation of the carbonyl group occurs the bromide ion is expelled due to the high reactivity of acyl bromide, it is a good leaving group. Go back to previous article. The function of the phosphorus is ultimately to convert a little of the acid into acid halide so it is the acid halide, not the acid itself, that undergoes this reaction. Learn how your comment data is processed. The resulting alpha-bromo acids can then be transformed into alpha-amino acids.

Completely I share your opinion. In it something is also I think, what is it good idea.