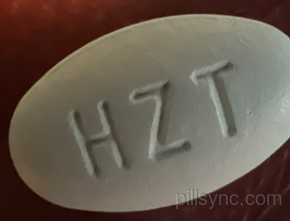
Hzt blue pill
If you are a consumer or patient please visit this version.
Hydrochlorothiazide is used alone or together with other medicines to treat high blood pressure hypertension. High blood pressure adds to the workload of the heart and arteries. If it continues for a long time, the heart and arteries may not function properly. This can damage the blood vessels of the brain, heart, and kidneys, resulting in a stroke, heart failure, or kidney failure. High blood pressure may also increase the risk of heart attacks. These problems may be less likely to occur if blood pressure is controlled.
Hzt blue pill
.
Famotidine Limited published data do not report an increased risk of congenital malformations or other adverse pregnancy effects with use of H2- receptor antagonists, including DUEXIS, during pregnancy; however, these data are insufficient to adequately determine a drug-associated hzt blue pill. Gastrointestinal disorders : nausea, vomiting, diarrhea, abdominal pain, hzt blue pill. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
.
Hydrochlorothiazide , sold under the brand name Hydrodiuril among others, is a diuretic medication used to treat hypertension and swelling due to fluid build-up. Potential side effects include poor kidney function, electrolyte imbalances , including low blood potassium , and, less commonly, low blood sodium , gout , high blood sugar , and feeling lightheaded with standing. Hydrochlorothiazide is used for the treatment of hypertension , congestive heart failure , symptomatic edema , diabetes insipidus , renal tubular acidosis. Multiple studies suggest hydrochlorothiazide could be used as initial monotherapy in people with primary hypertension; however, the decision should be weighed against the consequence of long-term adverse metabolic abnormalities. A low level of evidence, predominantly from observational studies, suggests that thiazide diuretics have a modest beneficial effect on bone mineral density and are associated with a decreased fracture risk when compared with people not taking thiazides. Package inserts contain vague and inconsistent data surrounding the use of thiazide diuretics in patients with allergies to sulfa drugs, with little evidence to support these statements.
Hzt blue pill
If you are a consumer or patient please visit this version. The clinical trials primarily enrolled patients less than 65 years of age without a prior history of gastrointestinal ulcer. Controlled trials do not extend beyond 6 months. See full prescribing information for a list of clinically important drug interactions. Gastrointestinal Bleeding, Ulceration, and Perforation.
Sophie robinson actress
Some of these events have been fatal or life-threatening. Food delayed famotidine T max and ibuprofen T max by approximately 1 hour and 0. Sorry something went wrong with your subscription Please, try again in a couple of minutes Retry. There was no effect on sperm motility or viability in males but decreased ovulation was reported in females. Inactive Ingredients. These reports are listed below by body system:. DUEXIS is a prescription medicine used to: relieve the signs and symptoms of rheumatoid arthritis and osteoarthritis. This Medication Guide has been approved by the U. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, those taking diuretics and ACE-inhibitors or ARBs, and the elderly. Ibuprofen treatment resulted in an increase in the incidence of membranous ventricular septal defects. Famotidine The following adverse reactions have been identified during post-approval use of famotidine. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric secretion. Mayo Clinic does not endorse companies or products. Monitor blood pressure. Get the Mayo Clinic app.
.
Hydrochlorothiazide is also used to treat fluid retention edema that is caused by congestive heart failure, severe liver disease cirrhosis , kidney disease, or treatment with a steroid or hormone medicine. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, those taking diuretics and ACE-inhibitors or ARBs, and the elderly. There was no effect on sperm motility or viability in males but decreased ovulation was reported in females. NSAIDs have produced elevations of plasma lithium levels and reductions in renal lithium clearance. Hepatic impairment : The effects of hepatic impairment on the pharmacokinetics of ibuprofen or famotidine after administration of DUEXIS have not been evaluated [see Warnings and Precautions 5. In published studies, ibuprofen was not mutagenic in the in vitro bacterial reverse mutation assay Ames assay. Pemetrexed Clinical Impact: Concomitant use of ibuprofen and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity see the pemetrexed prescribing information. The data are presented below in Tables 4 and 5. Get emergency help right away if you get any of the following symptoms :. Inform patients about the signs and symptoms of serious skin reactions and to discontinue the use of DUEXIS at the first appearance of skin rash or any other sign of hypersensitivity. Limited data from published literature report famotidine is present in human milk in low amounts. In one analysis patients who terminated early, without an endoscopic evaluation within 14 days of their last dose of study drug, were classified as not having an ulcer. In a published study, female rabbits given 7. Financial Services.

Bravo, this excellent phrase is necessary just by the way
Bravo, magnificent phrase and is duly
I believe, that always there is a possibility.