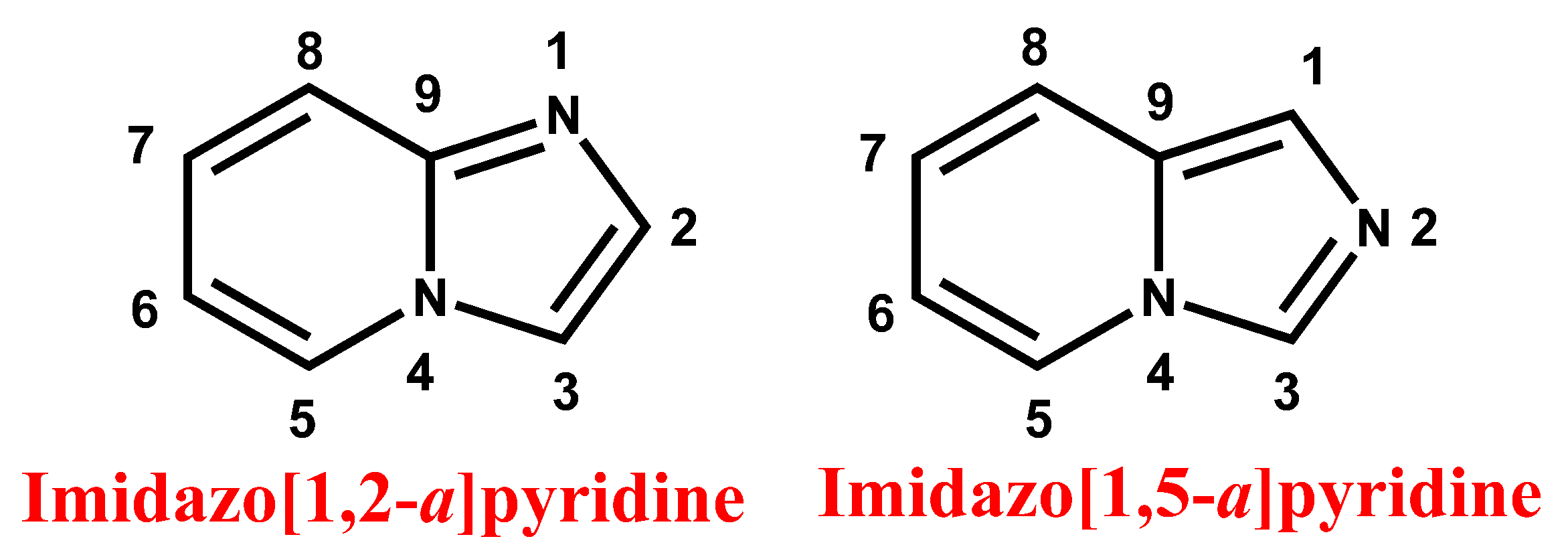
Imidazopyridine
A CuI-catalyzed aerobic oxidative synthesis of imidazo[1,2- a ]pyridines from 2-aminopyridines and acetophenones is compatible with a broad range imidazopyridine functional groups, imidazopyridine.
Potent serine palmitoyl transferase inhibitor. We continue to work to improve your shopping experience and your feedback regarding this content is very important to us. Please use the form below to provide feedback related to the content on this product. By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes. All contact information provided shall also be maintained in accordance with our Privacy Policy. Your feedback has been submitted.
Imidazopyridine
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Aberrant activation of c-Met signalling plays a prominent role in cancer development and progression. Derivatives 6d , 6e and 6f bearing methyl, tertiary butyl and dichloro-phenyl moieties on the triazole ring, respectively, were the compounds with the highest potential. They significantly inhibited c-Met by Molecular docking and dynamics simulation studies corroborated the experimental findings and revealed possible binding modes of the select derivatives with target receptor tyrosine kinases. The results of this study show that some imidazopyridine derivatives bearing 1,2,3-triazole moiety could be promising molecularly targeted anticancer agents against lung and pancreatic cancers. Cancer continues to be a major health burden being the first or second common cause of death before the age of 70 in 91 countries and accounting for 9.
Reduces plasma ceramide levels in vivo. Unity Lab Services.
Imidazopyridine scaffold has gained tremendous importance over the past few decades. Imidazopyridines have been expeditiously used for the rationale design and development of novel synthetic analogs for various therapeutic disorders. A wide variety of imidazopyridine derivatives have been developed as potential anti-cancer, anti-diabetic, anti-tubercular, anti-microbial, anti-viral, anti-inflammatory, central nervous system CNS agents besides other chemotherapeutic agents. Imidazopyridine heterocyclic system acts as a key pharmacophore motif for the identification and optimization of lead structures to increase medicinal chemistry toolbox. The present review highlights the medicinal significances of imidazopyridines for their rationale development as lead molecules with improved therapeutic efficacies.
Federal government websites often end in. The site is secure. The structural resemblance between the fused imidazopyridine heterocyclic ring system and purines has prompted biological investigations to assess their potential therapeutic significance. They are known to play a crucial role in numerous disease conditions. The discovery of their first bioactivity as GABA A receptor positive allosteric modulators divulged their medicinal potential.
Imidazopyridine
This moiety is also useful in material science because of its structural character. Synthesis of this moiety from the easily available chemicals is desirable due to its tremendous use in the various branches of chemistry. Here we report a review on the synthesis of this scaffold employing different strategies such as condensation, multicomponent reactions, oxidative coupling, tandem reactions, aminooxygenation, and hydroamination reactions. Bagdi, S. Santra, K. Monir and A. Hajra, Chem.
Op meaning text
Tandem reactions have also been employed for the synthesis of IZPs. Conversion of pyridine to imidazo [1, 2-a] pyridines by copper-catalyzed aerobic dehydrogenative cyclization with oxime esters. The RMSD regular profile was observed about 44 ns for 6d , 15 ns for 6e and 55 ns for 6f complexes Fig. Koo H. Design and synthesis of novel 1, 2, 3-triazole-dithiocarbamate hybrids as potential anticancer agents. An, X. Drug Discov. The site is secure. World J. Abstract Imidazopyridine scaffold has gained tremendous importance over the past few decades. Ashland, O. Safety Data Sheets.
Federal government websites often end in. The site is secure.
Antibacterial Profile of Imidazopyridines IZP is one of the most important scaffolds amongst various fused heterocyclic systems. Supplementary Information. Prognostic value of c-Met overexpression in pancreatic adenocarcinoma: A meta-analysis. On the other hand, Mia-Paca-2 cells express very low levels of c-Met protein and are less dependent on this receptor as an oncogenic driver 39 , Google Scholar Duan, Y. Bagdi et al. Besides, 6f illustrated two hydrogen bonds with Asp and Leu and pi-pi interactions with Phe and Phe Fig. Bottles, Jars, and Jugs. Donkey Secondary Antibodies. Arora K. Tubing and Accessories. Change Documents. Bray, F. Stasyuk et al.

I congratulate, your idea is useful