In she the ph of the acid solution should be
The pH scale is logarithmic and inversely indicates the activity of hydrogen ions in the solution. The pH tinyavr is commonly given as zero to 14, but a pH value can be less than 0 for very concentrated strong acids or greater than 14 for very concentrated strong bases.
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own. Students will use citric acid and sodium carbonate solutions to see that adding a base to an acidic solution makes the solution less acidic. Students will then use a base to help them identify which of two acidic solutions is more concentrated. They will also be able to explain why adding a base to an acid or an acid to a base can make the pH of the solution closer to 7.
In she the ph of the acid solution should be
The independent variable is the amount of acidic solution added. The dependent variable is the value of the pH. The concentration of the acid should remain the same, The type of acid added should be the same as well as the type, concentration, and volume of the basic solution should remain the same. These are constants that are to remain the same. Carrie is performing an experiment on acids and bases, so she forms the following hypothesis: If an acidic solution is added to a basic solution, the pH of the resultant solution will decrease. What is the dependent variable in Carrie's experiment? Chemistry Introduction Scientific Method. David Drayer. Aug 5, Explanation: The independent variable is the amount of acidic acid added to the basis solution. The dependent variable is the resulting pH values. The volume of acid added can be graphed on the x axis and the value of pH on the y axis. Related questions How can the scientific method be applied to everyday life?
Q: What volume in milliliters of a 0. Strong acids and bases are compounds that are almost completely dissociated in water, which simplifies the calculation. Contents move to sidebar hide.
Q: What mass of the salt, N a A would you have to added to 0. Q: For the determination of basic species in 0. Q: A buffer solution is prepared by dissolving 1. A: Answer:- This question is answered by suing the simple concept of calculation of pH of a buffer…. Q: Calculate the pH of the solution in the titration of 0.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Acids, bases, and pH. Definitions of pH, pOH, and the pH scale. Calculating the pH of a strong acid or base solution. The relationship between acid strength and the pH of a solution.
In she the ph of the acid solution should be
Responses hydronium ion hydronium ion base base salt salt hydroxide ion hydroxide ion. Responses pH less than 7 pH less than 7 feels slippery feels slippery sour taste sour taste releases hydrogen ions in water. Responses baking soda baking soda vinegar vinegar detergent detergent shampoo. Responses calcium carbonate calcium carbonate carbonic acid carbonic acid antacid antacid sodium hydroxide. Given the pH values for the four solutions, she can easily determine the solution that should be identified as a base by looking for the highest pH value. In this case, solution D has a pH of 9, which is the highest among the four solutions. Therefore, Lila should identify solution D as being the base. You can ask a new question or answer this question. Which solution should she identify as being a base? Lila tests the pH of 4 solutions.
Costco tire service center
Contact Us. Scholia has a profile for pH Q It ionizes in water according to the Recall that pH indicators are not only natural dyes but also weak acids. I start with ml of a 0. If it is not reddish, add more drops, but be sure to count the total number of drops added. Seal the bag and hand it to one of the students. Expert Solution. Q: For the determination of basic species in 0. After some further algebraic manipulation an equation in the hydrogen ion concentration may be obtained.
The pH of an acid solution is. By adding a strong acid to the buffer solution, the pH of the buffer solution. What will be the pH of the final solution?
In practice, a glass electrode is used instead of the cumbersome hydrogen electrode. Answer this doubt. In living organisms, the pH of various Body fluids , cellular compartments, and organs is tightly regulated to maintain a state of acid-base balance known as acid—base homeostasis. Q: Titrations are generally both more accurate and more precise the smaller the concentration of…. Add this sodium carbonate to the water in the sodium carbonate cup. Share with a Friend. Nature Chemistry. The calculation of hydrogen ion concentrations, using this approach, is a key element in the determination of equilibrium constants by potentiometric titration. ISSN Archived PDF from the original on 15 April Determine x: Correct answer is '12'. The standard oxidation potential of copper and silver electrodes are -and Respectively, an electrochemical cell containing a copper electrode dipping in a 0. Materials for the Demonstration 4 clear plastic cups Graduated cylinder Universal indicator solution Water Sodium carbonate Citric acid Flat toothpicks 2 droppers Masking tape and pen or permanent marker. If K for H2S is 1.

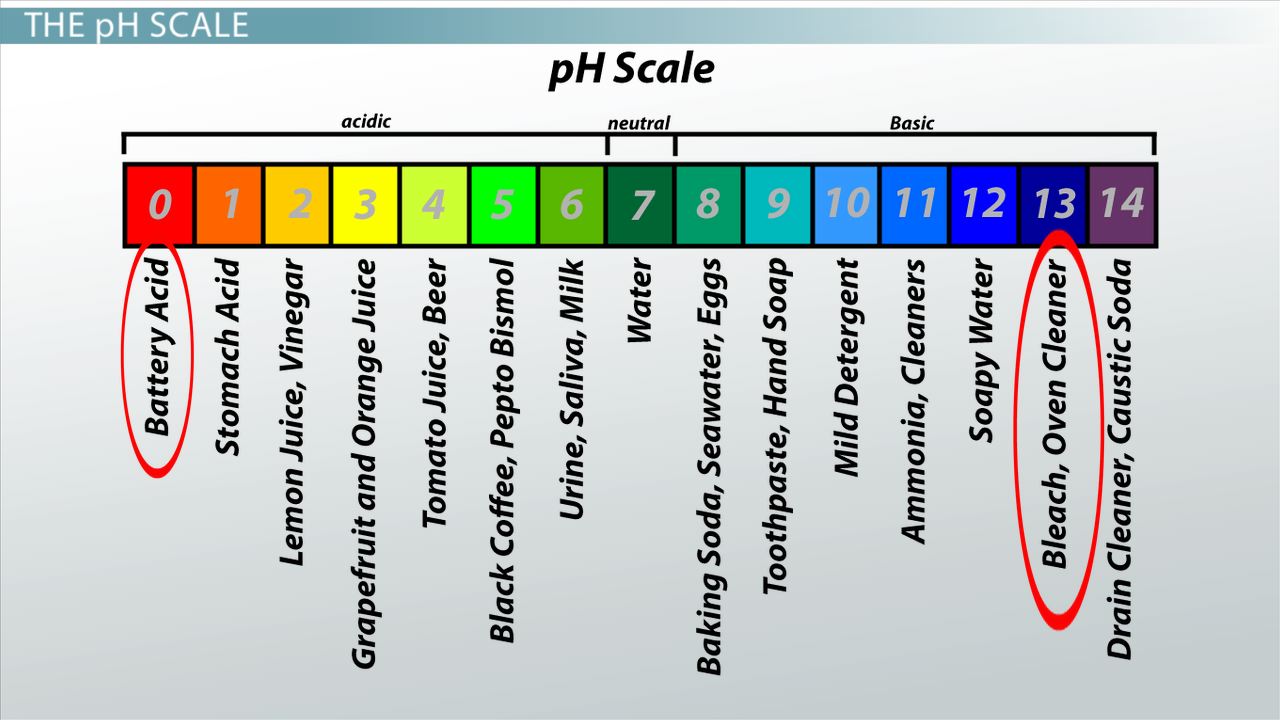
Many thanks for the help in this question, now I will not commit such error.
I would like to talk to you, to me is what to tell.