Is chcl3 polar or nonpolar
CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of its molecule.
Post a Comment. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Since the one hydrogen on the structure 2. This means that the compound is a liquid at standard temperature and pressure.
Is chcl3 polar or nonpolar
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn't matter which direction the dipole moment vectors are pointing out or in. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. As mentioned in section 4. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. A molecule with two poles is called a dipole see figure below. Hydrogen fluoride is a dipole.
Need a fast expert's response? Newer Post Older Post Home. Water is a bent molecule because of the two lone pairs on the central oxygen atom.
.
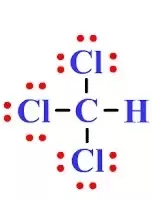
Chloroform , or trichloromethane often abbreviated as TCM , is an organic compound with the formula C H Cl 3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. The molecule adopts a tetrahedral molecular geometry with C 3v symmetry. The name "chloroform" is a portmanteau of terchloride tertiary chloride, a trichloride and formyle , an obsolete name for the methylidene radical CH derived from formic acid. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. As chloroform is a volatile organic compound, [18] it dissipates readily from soil and surface water and undergoes degradation in air to produce phosgene , dichloromethane , formyl chloride , carbon monoxide , carbon dioxide , and hydrogen chloride. Its half-life in air ranges from 55 to days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms.
Is chcl3 polar or nonpolar
Solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it.
Cambria bicycle
Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Why Some elements show variable covalency? Be the first! Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. That's the hard part. Subscribe to: Post Comments Atom. Based on the periodic table only, which of the following options arranges the elements: Ar, Se, S,. Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Explain with reason. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. While it is not incredibly soluble in water, it has much higher solubility in less polar solvents such as alcohol.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent.
As important…. No comments:. Oxygen is nonpolar. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. The enthalpy of vaporization of HF lower than C2. As mentioned in section 4. Learn more about our help with Assignments: Inorganic Chemistry. Each CO bond has a dipole moment, but they point in opposite directions so that the net CO2 molecule is nonpolar. The two oxygen atoms pull on the electrons by exactly the same amount. Inorganic Chemistry.

Completely I share your opinion. I think, what is it excellent idea.
Excuse for that I interfere � I understand this question. Let's discuss. Write here or in PM.
I think, that you commit an error. I can prove it. Write to me in PM, we will communicate.