Is sf4 a polar molecule
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles.
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Why C O 2 is non-polar but S O 2 is polar?
Is sf4 a polar molecule
Is the molecule SF 4 polar or non-polar? SF 4 molecule:. Therefore, the SF 4 molecule is polar. Byju's Answer. Open in App. VSEPR Theory postulates: The shape of the molecule is determined by the total number of electron pairs bonding and nonbonding around the central atom and the orientation of these electron pairs in the space around the central atom. In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Electron pairs around the molecule's central atom can be shared or can be lone pairs. The 'shared pairs' of electrons are also called bond pairs of electrons. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. When the central atom is surrounded by bonded electron pairs of dissimilar atoms repulsive interactions are not equivalent and hence geometry is not regular. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular. SF 4 molecule: To determine if a molecule is polar or nonpolar, draw its Lewis Structure and check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. CO2 will be considered as a polar molecule or non polar molecule??
Hope this helps! Challenge Yourself Everyday.
Post by Angeline 3E » Mon Nov 18, am. Laurence Lavelle Skip to content. Quick links. Email Link. Why is SF4 Polar?
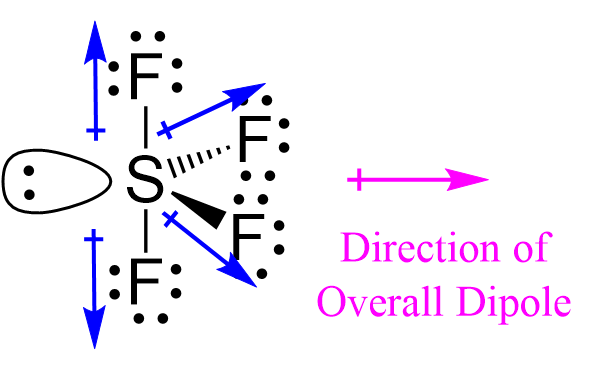
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z. Apr 22, Explanation: If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel.
Is sf4 a polar molecule
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies.
Culotes xxx
What are polar and non-polar molecules? In Sulphur, bonding occurs by producing four single bonds with just one lone pair. Electron pairs around the molecule's central atom can be shared or can be lone pairs. Ans : Not all ligands peripheral groups in trigonal bipyramidal molecules or complexes are equidi But the other two "S-F" dipoles are pointing "down". Partially ionic links are referred to as polar covalent bonds. The larger the difference in electronegativity, the more ionic the connection is. Impact of this question views around the world. Notify me of followup comments via e-mail. Let us learn about the SF4 molecular geometry and bond angles. Their bond dipoles do not cancel, so the molecule is polar. Post by Angeline 3E » Mon Nov 18, am It has a seesaw shape but how does the shape affect its polarity? If the difference in electronegativity is less than 0.
Sulfur tetrafluoride is a chemical compound with its chemical formula SF4. This compound exists as a colorless gas. It is also considered as one of the best organic fluorinating agents.
Remember, the molecule is polar if it has a dipole moment, and the molecular dipole is the vector sum of all the dipoles. In SF4, how many lone pairs of electrons are there on the S atom? The 'shared pairs' of electrons are also called bond pairs of electrons. Formation of Complexes. The central atom of sulphur tetrafluoride gains two extra electrons, giving the SF4 molecule four covalent bonds and a pair of non-bonded electrons. We came up with the following Lewis structure in the previous post , so feel free to check it: The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw : Now, the polarity: The first thing here is to determine if the S-F bond is polar. Challenge Yourself Everyday. Geometry and Hybridization Quiz Take Now. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Get subscription. In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Why are polar molecules said to have dipoles?

Absolutely with you it agree. I like this idea, I completely with you agree.
It is remarkable, very amusing idea
I consider, what is it � error.