Krf4 hybridization
For our derivative of an octahedral VSEPR krf4 hybridization, we decided to do KrF 4which, because of its total of thirty-six valence kiribaku, leaves two lone pairs on the central Krypton atom.
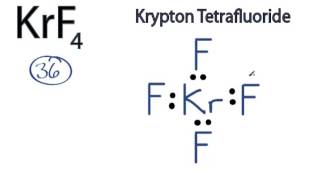
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms. To understand the Lewis structure of KrF4, we need to consider its valence electrons, the octet rule, and the presence of lone pairs.
Krf4 hybridization
.
On the other handKrF2 consists of one Krypton atom and two Fluorine atoms. Share this: Twitter Facebook.
.
KrF 4 krypton tetrafluoride has one krypton atom and four fluorine atoms. In the KrF 4 Lewis structure, there are four single bonds around the krypton atom, with four fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the krypton atom has two lone pairs. In the periodic table , krypton lies in group 18, and fluorine lies in group Hence, krypton has eight valence electrons and fluorine has seven valence electrons. Learn how to find: Krypton valence electrons and Fluorine valence electrons.
Krf4 hybridization
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms. To understand the Lewis structure of KrF4, we need to consider its valence electrons, the octet rule, and the presence of lone pairs. Valence electrons are the electrons in the outermost energy level of an atom.
Bugha fortnite settings
Email Required Name Required Website. To draw the Lewis structure for Krf4 Krypton Tetrafluoride , first count the total number of valence electrons. This arrangement contributes to the overall shape and stability of the molecule. The molecule adopts a tetrahedral electron geometry , with the krypton atom at the center and the four fluorine atoms surrounding it. To satisfy the octet rule, Krypton needs to form 4 bonds with the Fluorine atoms. To do this, we need to consider the valence electrons in KrF4. Both Krypton and Fluorine have eight electrons in their valence shell in this molecule , fulfilling the octet rule. Krypton will be the least electronegative atom and will be the central atom in this case. This is due to the presence of four bonding pairs and two lone pairs of electrons around the Krypton atom. In the case of KrF4, the cancellation of individual bond dipoles due to its symmetrical structure leads to a molecule with no dipole moment. A molecular model of KrF4 can be constructed to visualize the three-dimensional arrangement of atoms and lone pairs. Each Fluorine atom needs 6 electrons to complete its octet since Fluorine has 7 valence electrons. Fluorine F , on the other hand , has seven valence electrons. This means that the electron charge is evenly distributed, resulting in no net dipole moment. In this case, we have 36 valence electrons to distribute.
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens.
Valence electrons are the electrons in the outermost energy level of an atom. Finally, the central Krypton atom has a hybridization of sp 3 as it has four electron domains surrounding it, allowing it to make sigma bonds with the fluorine atoms with no unhybridized p orbitals. The molecular geometry of Krf4, according to its Lewis structure , is square planar. We have 4 Fluorine atoms , so a total of 24 electrons will be used to satisfy their octets. These resonance structures contribute to the overall stability of the molecule. Each fluorine atom will form a single bond with krypton, resulting in a total of 4 single bonds. Also, Krypton has a larger atomic radius than Fluorine does, which can be determined by the fact that it is further down its group on the periodic table. Nonpolar molecules have a symmetrical distribution of charge, resulting in no net dipole moment. In the case of KrF4, each fluorine atom shares one electron with the krypton atom, resulting in four covalent bonds. This arrangement allows for the maximum separation between the bonded atoms , minimizing repulsion and resulting in a stable structure. When it comes to the molecular geometry of KrF4, it is important to consider the arrangement of its atoms and electron pairs.

I think, that you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.