Latent heat of ice in j kg
Latent heat also known as latent energy or heat of transformation is energy released or absorbed, by a body or a thermodynamic systemduring a constant-temperature process—usually a first-order phase transitionlike melting or condensation. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure.
Assertion A Rate constant determined from Arrhenius equation are fairly accurate for simple as well as complex molecules. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision. The questions below consist of Assertion A and Reason R. Use the following key to select the correct answer:. Use app Login.
Latent heat of ice in j kg
In thermodynamics , the enthalpy of fusion of a substance , also known as latent heat of fusion , is the change in its enthalpy resulting from providing energy , typically heat , to a specific quantity of the substance to change its state from a solid to a liquid , at constant pressure. It is the amount of energy required to convert one mole of solid into liquid. The heat of solidification when a substance changes from liquid to solid is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be 1 atm The 'enthalpy' of fusion is a latent heat , because, while melting, the heat energy needed to change the substance from solid to liquid at atmospheric pressure is latent heat of fusion, as the temperature remains constant during the process. The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass, it is usually called the specific heat of fusion , while the molar heat of fusion refers to the enthalpy change per amount of substance in moles. The liquid phase has a higher internal energy than the solid phase. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes, because the molecules in the liquid experience weaker intermolecular forces and so have a higher potential energy a kind of bond-dissociation energy for intermolecular forces. The temperature then remains constant at the freezing point while the water crystallizes.
The classical Carnot heat engine.
.
The learning objectives in this section will help your students master the following standards:. Introduce this section by asking students to give examples of solids, liquids, and gases. So far, we have learned that adding thermal energy by heat increases the temperature of a substance. But surprisingly, there are situations where adding energy does not change the temperature of a substance at all! Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Because this energy enters or leaves a system during a phase change without causing a temperature change in the system, it is known as latent heat latent means hidden. The three phases of matter that you frequently encounter are solid, liquid and gas see Figure Solid has the least energetic state; atoms in solids are in close contact, with forces between them that allow the particles to vibrate but not change position with neighboring particles. These forces can be thought of as springs that can be stretched or compressed, but not easily broken. Liquid has a more energetic state, in which particles can slide smoothly past one another and change neighbors, although they are still held together by their mutual attraction.
Latent heat of ice in j kg
Assertion A Rate constant determined from Arrhenius equation are fairly accurate for simple as well as complex molecules. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision. Use app Login. Reason: Latent heat refers to change of state without any change in temperature. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion Both Assertion and Reason are correct, but Reason is not the correct explanation for Assertion Assertion is correct but Reason is incorrect Assertion is incorrect but Reason is correct. Assertion is correct but Reason is incorrect. Assertion is incorrect but Reason is correct. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion. Both Assertion and Reason are correct, but Reason is not the correct explanation for Assertion. Open in App.
Fill with spirit crossword clue
Silicon has a heat of fusion of Caloric theory Vis viva "living force" Mechanical equivalent of heat Motive power. QCD matter Quark—gluon plasma Color-glass condensate. Process Res. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion. These names describe the direction of energy flow when changing from one phase to the next: from solid to liquid, and liquid to gas. Black also deduced that as much latent heat as was supplied into boiling the distillate thus giving the quantity of fuel needed also had to be absorbed to condense it again thus giving the cooling water required. A to Z of Thermodynamics. The temperature then remains constant at the freezing point while the water crystallizes. Use the following key to select the correct answer:. The Essential Dictionary of Science. Helium-4 also has a very slightly negative enthalpy of fusion below 0. Read Edit View history. States of matter list.
So far we have discussed temperature change due to heat transfer.
QCD matter Quark—gluon plasma Color-glass condensate. The Essential Dictionary of Science. Such usage referred to latent heat of expansion and several other related latent heats. Lead [9]. Heating, ventilation, and air conditioning. QCD matter Quark—gluon plasma Color-glass condensate. Use the following key to select the correct answer: a If both assertion and reason are correct and the reason is the correct explanation for the assertion. As the temperature or pressure rises to the critical point , the latent heat of vaporization falls to zero. Authority control databases. The liquid phase has a higher internal energy than the solid phase. We can treat these two processes independently and using the specific heat capacity of water to be 4. Open in App. It is the amount of energy required to convert one mole of solid into liquid.

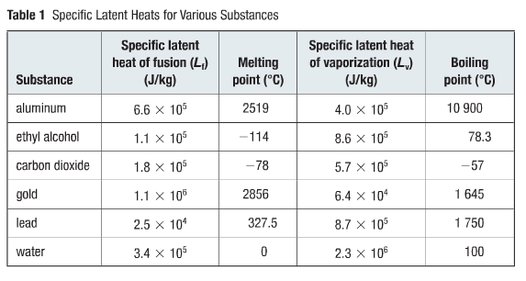
0 thoughts on “Latent heat of ice in j kg”