Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is. Q: Q2. Draw Lewis structure of the following species, indication formal charges and resonance where….
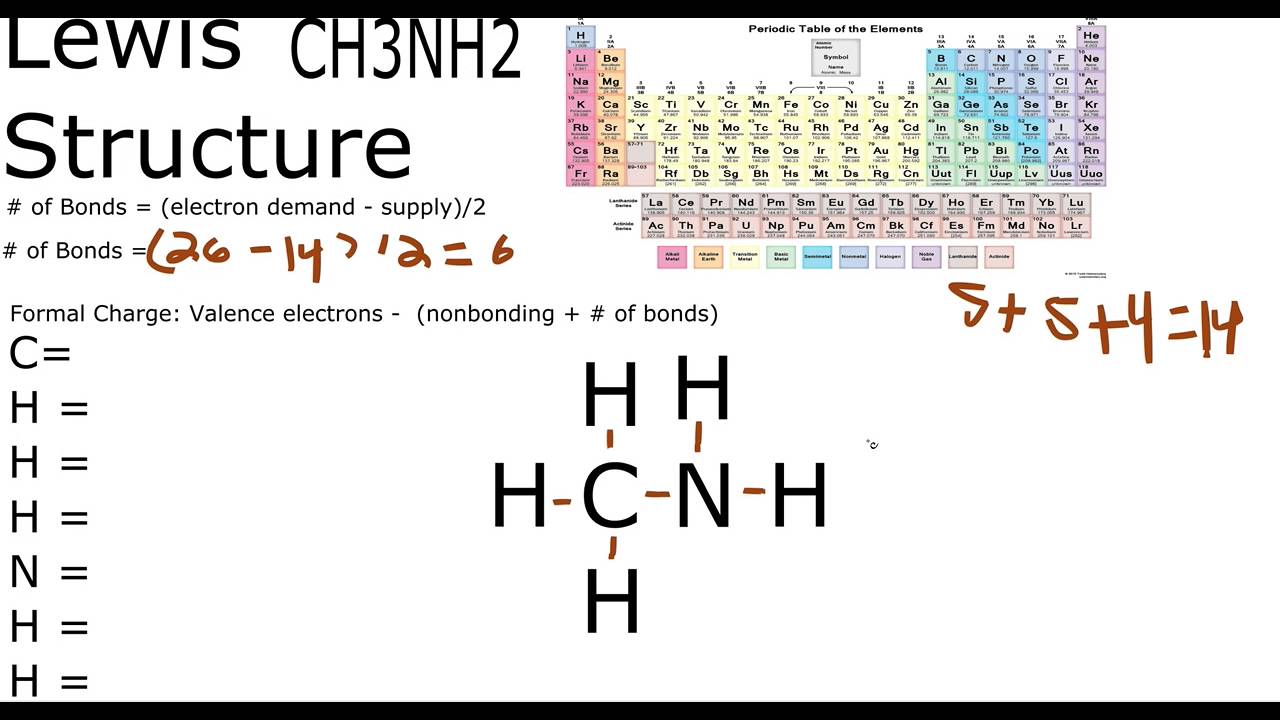
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Nitrogen is a group 15 element on the periodic table.
Lewis diagram for ch3nh2
.
Now, you can see the electronegativity values of carbon atom C and nitrogen atom N in the above periodic table. Principles of Modern Chemistry 8th Edition. Trending now This is a popular solution!
.
CH 3 NH 2 methylamine has one carbon atom, five hydrogen atoms, and one nitrogen atom. The carbon atom is attached with three hydrogen atoms, and the nitrogen atom is attached with two hydrogen atoms. And on the nitrogen atom, there is one lone pair. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and nitrogen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and nitrogen has five valence electrons.
Lewis diagram for ch3nh2
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair. Methylamine is an organic molecule that is colorless in the gaseous state and is a derivative of ammonia. Moreover, this molecule has a strong pungent fishy smell and is used commercially to produce ephedrine, carbofuran, metham sodium, methyl formamide, theophylline, carbaryl, N-methyl pyrrolidone. Methylamine is a known nucleophile where it bonds with the electrophiles by donating the electron pairs, which makes it a Lewis base. A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration.
Ats mod
Q: What is the central atom of SiSe2, how many lone pairs does it have, and how many single and double… A:. Which atom in this structure…. Q: Problem 62P: For each of the following molecules or molecular ions, give the steric number, sketch and name the Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom? Solved in 3 steps with 4 images. Knowledge Booster. Problem 71P: Assign oxidation numbers to the atoms in each of the following species Daniel L. Q: Estimate the enthalpy change AH of the following reaction using the average bond energies in the… A:. Pat Gillis, Laurie J.
Ready to learn how to draw the lewis structure of CH3NH2?
Q: For each skeletal structure below, satisfy the valences or octets of all of the atoms by filling…. Q: What is the central atom of SiSe2, how many lone pairs does it have, and how many single and double… A:. Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. A: Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures,…. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. Q: hat would be the formal charge on Se and S and both Os for the most stable resonance structures of… A: The stable resonance form of the given species can be illustrated as follows:. Problem 40P: Assign formal charges to all atoms in the following Lewis diagrams. Q: Octogen, most commonly referred to by the abbreviation HMX is an explosive. Justify your answer in terms of dipole moments and geometry. A: The formal charge is a total charge existing on an atom in a molecule. Problem 49P: Under certain conditions, the stable form of sulfur consists of rings of eight sulfur atoms.

At me a similar situation. I invite to discussion.
Quite right! It is good idea. It is ready to support you.
I do not know.