Lewis diagram for hcooh
Submitted by Jennifer W. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators.
Lewis Dot Structures. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of HCN. Is the octet roule obeyed in these structures? Write the Lewis dot structure for the following covalent molecules: S i H 4. Can a non-polar molecule have polar covalent bonds? Give one example each for a compound with a an ionic bond b a cov
Lewis diagram for hcooh
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. Q: Choose the letter of the correct answer 1. What type s of intermolecular forces can exist C2H6 and…. A: Since you have asked multiple question, we will solve the first question for you. If you want any…. Q: A buffer solution contains 0. Q: What is the final concentration of nitrate ion What is the final concentration of sodium ion. A: To calculate final concentration of ion, we would first their moles and using total volume of water…. Calculate the pH of 1. A: Given, 0. Volume 1. Which of the following is the reason for the fact that antacids have to be chewed before….
Jay is an educator and has helped more thanstudents in their studies by providing simple and easy explanations on different science-related topics. In the presence of oxygen gas and a….
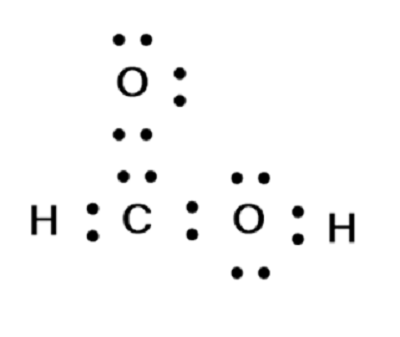
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Carbon is group 14 element on the periodic table.
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Carbon is a group 14 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the HCOOH molecule, if we compare the carbon atom C , oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than oxygen and carbon. But as per the rule, we have to keep hydrogen outside.
Lewis diagram for hcooh
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 18 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
German porn stars nude
You can see you have a double bond right here, that's your double bond there. Count the total number of valence electrons: H has 1, C has 4, O has 6, and there are two O atoms and two H atoms. Which of the following is the reason for the fact that antacids have to be chewed before… A: Chemical reaction can be defined as the branch of chemistry that deals with rates of chemical…. Convert moles of white and brown sugar to grams and then to cups using the conversion, 1 cup of… A:. And four bonds are already marked. A: Since you have asked multiple question, we will solve the first question for you. And both carbon and oxygen are the period 2 elements , so they can not keep more than 8 electrons in their last shell. So for top oxygen, there are three lone pairs, for right oxygen, there are two lone pairs, and for carbon, there is zero lone pair because all five electron pairs are over. So we have 8, 10, 12, 14, 16, and 18 valence electrons. Let's put the Carbon at the center; and we have this H here, let's put it out here; and then we have two Oxygens. Make sure the formal charges on each atom sum up to zero. Gender Male Female Others. View in App Not Now. So we have to only mark the remaining five electron pairs as lone pairs on the sketch.
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes.
And then let's go around the outer atoms and complete the octets. Formal Charges. How many sigma- and pi- bonds are present in naphthalene? View all answers and join this discussion on the EduRev App. Q: Choose the letter of the correct answer 1. Get Better Grades Now. Characterize the types of bonds in terms of electronegativity difference. What do you understand by bond pairs and lone pairs of electrons? University of Ghana Hi, my name is Derrick, and I enjoy teaching and sharing what I know with others. Step by step Solved in 2 steps with 1 images. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Therefore, reduce the charges as below by converting lone pairs to bonds. Search for:.

It seems to me it is excellent idea. I agree with you.
No doubt.