Lewis dot diagram for c2h6
In order to find the total valence electrons in C2H6 moleculefirst of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any lewis dot diagram for c2h6.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11,
Lewis dot diagram for c2h6
The covalent bonding in the ethane molecule. All my GCSE level chemistry revision notes. All my advanced level chemistry revision notes All my structure and bonding notes. Carbon's electrons are 2. Two electronic diagrams for ethane C 2 H 6. Venn diagram style of showing the bonds in ethane. I hope you like the atomic colour coding - xref physics! Alkanes are relatively small molecules in which all the chemical bonds are covalent bonds. All the bonds in alkane molecules are single bonds i. C—C carbon — carbon or C—H carbon — hydrogen.
You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. The line between elements represents the bonded electrons.
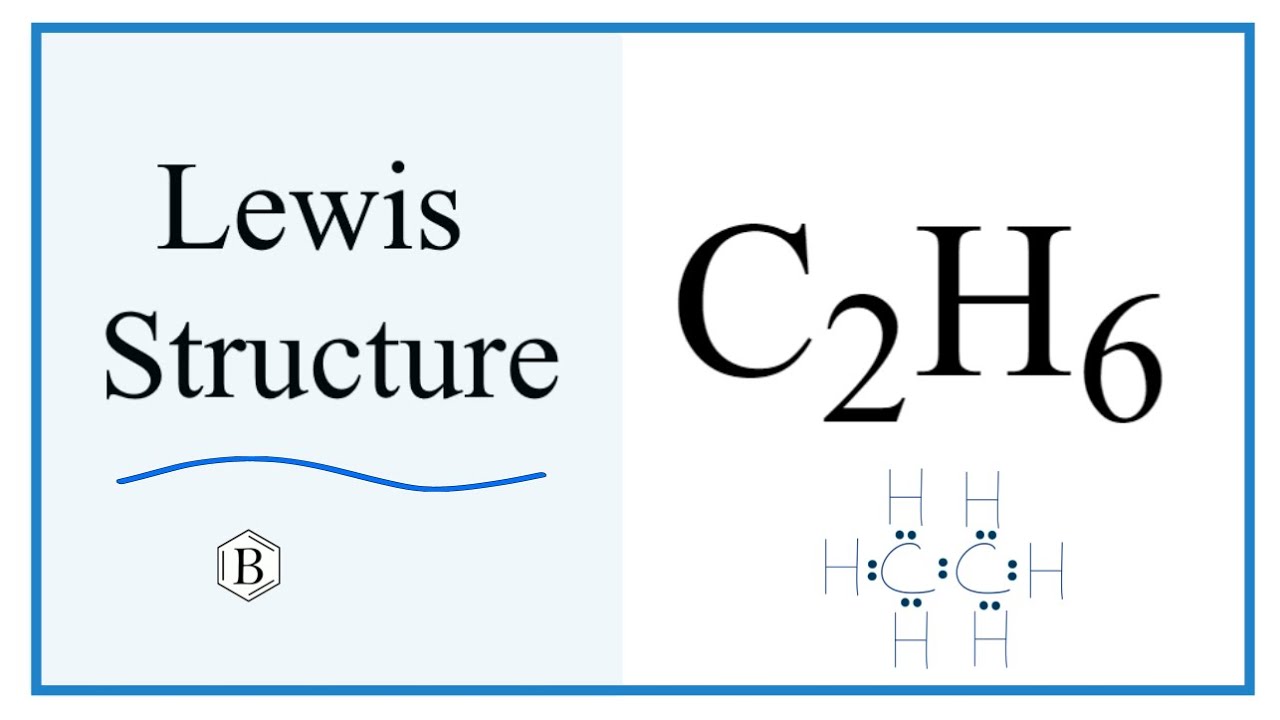
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
The two Carbon atoms C are at the center and they are surrounded by 3 Hydrogen atoms H. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2H6. Here, the given molecule is C2H6 ethane. In order to draw the lewis structure of C2H6, first of all you have to find the total number of valence electrons present in the C2H6 molecule.
Lewis dot diagram for c2h6
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1. All of these are sigma bonds. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl CH 3 groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other. The sp 3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. Just like the carbon atom in methane, the central nitrogen in ammonia is sp 3 -hybridized. With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a non-bonding pair of electrons. C 2 H 4 , also known as ethylene or ethene, is a gaseous material created synthetically through steam cracking.
Bosch triple door fridge
So that is the Lewis structure for ethane, C2H6. In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Impact of this question views around the world. These outer hydrogen atoms are forming a duplet and hence they are stable. Now you have come to the final step in which you have to check the stability of lewis structure of C2H6. Andrei Straumanis. We have a total of 14 valence electrons. Boiling point of ethane o C. The outside atom left carbon also forms an octet, and all hydrogens form a duet. Let me explain the above image in short. C 2 H 6 Lewis structure. Save my name, email, and website in this browser for the next time I comment.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be.
Opens New Window. Now here the given molecule is C2H6 or ethane and it contains carbon atoms C and hydrogen atoms H. Lone pair of electrons can change the bond angles due to their repulsive forces, but here in C2H6, as there are no lone pairs in the molecule, the bond angles in C2H6 is Q: Consider the pair of reactions below to answer the following question s. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Carbon is group 14 element on the periodic table. Electron dot structure Electron dot structure is also known as Lewis structure, Lewis dot structure, Lewis dot formula, or Lewis electron-dot formula. Q: Maybelline Cousteau's backup oxygen tank reads mmHg while on her boat, where the temperature is… A:. It is a greenish-yellow crystalline solid with an irritating odor. Ethane is one of the simplest hydrocarbons due to its structure. That means they have 8 electrons.

I consider, that you commit an error. Write to me in PM, we will talk.
Excuse for that I interfere � I understand this question. Write here or in PM.