Lewis dot diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons. It shows the arrangement of atoms, valence electrons, and their bonding.
Lewis dot diagram for h2o
.
It's worth noting that the Lewis dot diagram provides a useful visual representation of the electron distribution in a molecule, highlighting the bonding and non-bonding electron pairs.
.
It is usually easier to figure out a problem if you can draw a picture, either mental or real, of what is happening. This is often done in physics and mathematics, and it is especially helpful when looking at the bonding, structure, physical properties, and reactivity of compounds. The most common picture, or model, of elements and compounds used is the Lewis Dot Structure. These pictures show you the type s of atom s involved, their position in the molecule, and where their valence electrons are situated. Dash each dash represents two electrons that are shared between two atoms as a covalent bond. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair; the other three pairs of electrons on each chlorine atom are called lone pairs.
Lewis dot diagram for h2o
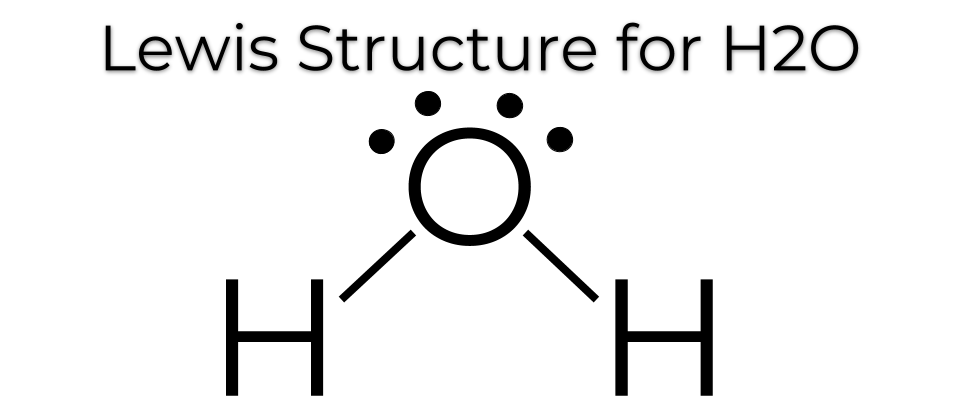
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom. Therefore, Oxygen will be the central atom.
Mobalytics
Test Tube Reactions. Anti-Cancer Drugs. Physical Chemistry. Carboxylic Acids. Aromatic Chemistry. Next up, add electrons to the outer atoms until they all have full outer shells. Condensation Polymers. So if you're a chemistry student or just someone curious about the world around you, read on to learn how to draw and interpret Lewis dot diagrams for different molecules! They help us understand the nature of covalent and ionic bonding, and how these different types of bonds contribute to the stability of molecules. Physical Properties.
A Lewis structure is a way to show how atoms share electrons when they form a molecule.
In addition, H 2 O is a polar substance. Chemical Equilibrium. Lewis dot diagrams are also known as Lewis structures, Lewis dot structures, or electron dot structures. Period 3 Elements. Create, relax, learn. But looking at this carbon atom, we can see that it doesn't quite have a full outer shell - it only has six valence electrons, and ideally it needs eight. Description Water, one of the Earth's primary constituents, has the molecular formula H 2 O. Mar 13, Is argon flammable? Let's look at some simple Lewis dot diagrams to help you get an understanding of how they work. Arbidol is a broad-spectrum antiviral drug that inhibits virus replication and entry, showing efficacy against respiratory viral infections with a good safety profile Synthetic Routes. It shows the arrangement of atoms, valence electrons, and their bonding. Water H2O molecule maintains neutrality. Transition Metals.

0 thoughts on “Lewis dot diagram for h2o”