Lewis dot structure of ethanol
On the left, we have the Oxygen atom between the two Carbons. This is called dimethyl ether. On the right, the Oxygen atom's on the outside with the Hydrogen attached to it.
Wiki User. Tetra Hedral. Phosphorus pentabromide:. I uploaded a jpg of the acetate ion Lewis structure to imageshack. Just click the "related link" below and you should see it. Many people draw Lewis Structures with minor variations, but this should give you the basic idea.
Lewis dot structure of ethanol
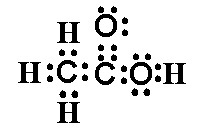
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. Next to draw the Lewis dot structure for ethanol , the alcohol which is found in fermented beverages. Fermentation is the organic chemical reaction whereby yeast metabolize sugars which are found in fruits to ethanol and carbon dioxide. The molecular formula for ethanol is C 2 H 6 O. There would be 2 ways of writing the Lewis structural formula C 2 H 6 O. When compounds have the same molecular formula but different structural formulas they are referred to as isomers. We want to draw the Lewis dot structure for ethanol. Another way of writing the molecular formula for ethanol helps to illustrate exactly which isomer we are referring to and how we should draw the Lewis dot structure. Writing the formula in this way CH 3 —CH 2 —OH tells us which of the structural formulas we are referring to and how to draw the Lewis dot structure. When we draw the Lewis dot structure, we first write the central atoms.
I uploaded a jpg of the acetate ion Lewis structure to imageshack. They fill the outer shells for each of the atoms in the C2H6O structure.
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures.
The oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2H5OH. Here, the given molecule is C2H5OH ethanol. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table.
Lewis dot structure of ethanol
Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt. It is also used in laboratories for synthesis of other organic compounds and is often stored in wash bottles to use it as a solvent. Now we shall learn about the Lewis structure of this molecule for a better understanding of its physical and chemical properties.
Twinkgetsnaked
An electron is represented by a dot. So they're both valid Lewis structures. See the Big List of Lewis Structures. One electron is needed to complete a fluorine octet. And then the Oxygens, they also have eight valence electrons, so they have—their outer shells are full, as well. Although it has the same molecular formula as ethanol, it is a different compound with different physical properties because of the different way the atoms are arranged. Lewis structures are useful for understanding chemical bonding. Dimethyl ether is a gas, not a liquid. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. Opens New Window. It is an acetate conjugate acid. When was Lewis structure created? Follow: RSS Twitter. Next step is to place dots for the valence electrons. Fermentation is the organic chemical reaction whereby yeast metabolize sugars which are found in fruits to ethanol and carbon dioxide.
Lewis used simple diagrams now called Lewis diagrams to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.
The Hydrogens all have two valence electrons, and that's all they need to have a full outer shell. Here is the Lewis dot structure for the other isomer of C 2 H 6 O, dimethyl ether. The chemical structure of ethanol consists of? The Lewis structure is complete when we connect the dots to show the sharing of electron pairs between different atoms. Remember hydrogens always go on the outside. Although it has the same molecular formula as ethanol, it is a different compound with different physical properties because of the different way the atoms are arranged. Englesh kumar permalink. Besides regulating the acidity of food, it is also used as an antimicrobial food preservative and a Daphnia magna metabolite. The sulphur atoms also have two lone pairs. On the left, we have the Oxygen atom between the two Carbons.

0 thoughts on “Lewis dot structure of ethanol”