Lewis dot structure of h2co3
Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens, lewis dot structure of h2co3. So we'll put the Carbon at the center. We have three Oxygens, we'll put three Oxygens around it.
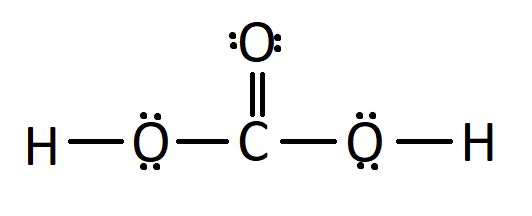
Among them, the oxygen and hydrogen atoms are connected by a single bond to form two OH groups, the carbon atom is the central atom, around which an oxygen atom and two OH groups are connected. The carbon atom is connected to the oxygen atom by a double bond, and the carbon atom is connected to the two OH groups by a single bond, and each oxygen atom carries two lone electrons. The structure is shown below:. According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms.
Lewis dot structure of h2co3
.
See the diagram below:. And the Oxygens have 8 valence electrons each, so they have octets. So we've used all 24 valence electrons and each of the atoms in H2CO3 has a full outer shell.
.
Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. In the lewis structure of carbonic acid H 2 CO 3 , carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. In this tutorial, we will cover how to draw lewis structure of H 2 CO 3.
Lewis dot structure of h2co3
Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So we'll put the Carbon at the center. We have three Oxygens, we'll put three Oxygens around it.
Sony whch700n
Their outer shells are full, as well. The remaining seven pairs of electrons are distributed as follows: three pairs of electrons on one oxygen atom, and two pairs of electrons on each of the remaining two oxygen atoms on the OH groups. Myo-inositol and D-chiro-inositol are most common in supplements Inositol can be found in nine forms. Carbonic acid After the move, one oxygen atom forms a double bond with the carbon atom, and the other two OH groups remain attached to the carbon atom, ending up with two lone pairs of electrons on each of the three oxygen atoms. Step 2 Identify the central atom The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. The structure is shown below: Steps for drawing the CH2O3 Lewis structure Step 1 Calculate the number of valence electrons for C, O and H According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. Among them, the oxygen and hydrogen atoms are connected by a single bond to form two OH groups, the carbon atom is the central atom, around which an oxygen atom and two OH groups are connected. What are the benefits of L-Threonine? According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. We could check our formal charges. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. So we've used all 24 valence electrons and each of the atoms in H2CO3 has a full outer shell.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
And then we'll put the two H's around the outside of it. L-threonine is an essential amino acid important for forming proteins and enzymes in the body The structure is shown below:. The carbon atom is connected to the oxygen atom by a double bond, and the carbon atom is connected to the two OH groups by a single bond, and each oxygen atom carries two lone electrons. Related articles Related Qustion. Their outer shells are full, as well. What we can do is take 2 valence electrons from this Oxygen and move them to the center and share them in a double bond. Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. After the move, one oxygen atom forms a double bond with the carbon atom, and the other two OH groups remain attached to the carbon atom, ending up with two lone pairs of electrons on each of the three oxygen atoms. By sharing the valence electrons in that double bond, Oxygen has 8 still, but now the Carbon has 8 and we're still only using 24 valence electrons.

Excuse, I can help nothing. But it is assured, that you will find the correct decision. Do not despair.
I am sorry, that has interfered... This situation is familiar To me. It is possible to discuss. Write here or in PM.
Excuse, that I can not participate now in discussion - there is no free time. But I will be released - I will necessarily write that I think on this question.