Lewis structure k2s
Submitted by William D. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, lewis structure k2s, and study partner. Ask unlimited questions and get video answers from our expert STEM educators.
Potassium sulfide represented by the chemical formula K 2 S is a compound of potassium and sulfur that is moderately soluble in acids [1]. It is deliquescent and may spontaneously ignite in air. It is a reducing agent and an ionic compound [4]. Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS. Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9]. Potassium sulfide reacts with cobalt iii bromide to produce cobalt iii sulfide and potassium bromide [10]. Potassium sulfide reacts with dilute hydrochloric acid to produce potassium chloride and hydrogen sulfide [11].
Lewis structure k2s
Chapter 1 Chapter 1: The Chemical World 1. In Section 4. The astute reader may have noticed something: many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it, so the atom becomes positively charged. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The octet rule explains the favorable trend of atoms having eight electrons in their valence shell. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make. It is not impossible to violate the octet rule. Consider sodium: in its elemental form, it has one valence electron and is stable. The octet rule is a result of trends in energies and is useful in explaining why atoms form the ions that they do.
Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions, lewis structure k2s.
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Questions Courses. Do you need an answer to a question different from the above?
Potassium sulfide represented by the chemical formula K 2 S is a compound of potassium and sulfur that is moderately soluble in acids [1]. It is deliquescent and may spontaneously ignite in air. It is a reducing agent and an ionic compound [4]. Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS. Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9]. Potassium sulfide reacts with cobalt iii bromide to produce cobalt iii sulfide and potassium bromide [10]. Potassium sulfide reacts with dilute hydrochloric acid to produce potassium chloride and hydrogen sulfide [11].
Lewis structure k2s
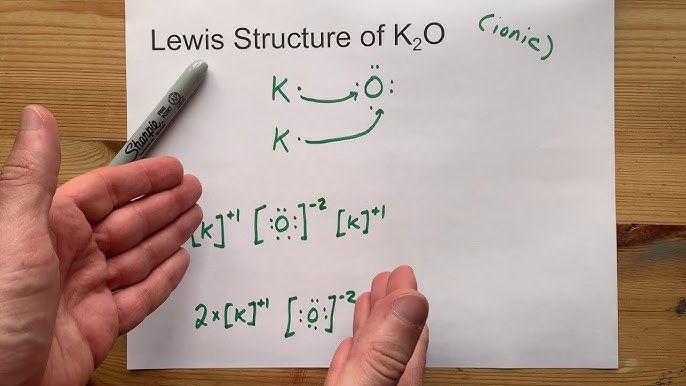
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures. This section will discuss the rules for writing out Lewis structures correctly. Writing out Lewis structures can be at times, tricky and somewhat difficult. A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like.
Same day detox for all drugs
Do you need an answer to a question different from the above? The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Trending Topics Mass Spectrometry. Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9]. Try it in the Numerade app? All Rights Reserved. Amorphous Polymers. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make. Potassium Sulfide Formula. Time is being
Chapter 1 Chapter 1: The Chemical World 1. In Section 4.
Question 2 What is a commonly held and persistent assumption about Nursing? Still have questions? Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Define ionic bond. HCFO d. Be sure to consider Resources Leaderboard All Tags Unanswered. Tell us more Hide this section if you want to rate later. Video Answer. I came second at the state level of a Physics Olympiad when I was in secondary school. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make. This is required by the law of conservation of matter as well. Gender Stereotype In discussing this question Ask unlimited questions and get video answers from our expert STEM educators. Best Answer.

What good interlocutors :)