Lewis structure of h2so3
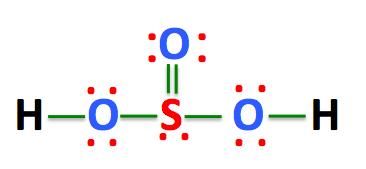
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H lewis structure of h2so3 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule.
Submitted by Christopher J. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Choose the correct Lewis structure for the oxyacid H2SO3, called sulfurous acid. A skeletal structure for sulfurous acid H2SO3 is shown below. Starting from this structure, complete the Lewis structure that follows the octet rule on all atoms.
Lewis structure of h2so3
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur. Oxyacids of sulphur are classified into three series.
Besides its uses, sulfurous lewis structure of h2so3 causes some health hazards. And the other two O-atoms form two double or coordinate bonds with the S-atom. In simple words, it can be said to be one of the oxyacids of sulphur.
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable. Q: Write Lewis structures of simple molecules following the octet rule.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Lewis structure of h2so3
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so…. Learn how to find: Hydrogen valence electrons , Sulfur valence electrons , and Oxygen valence electrons. We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Imágenes de buenos días gratis para whatsapp
When it oxidises a certain substance, it is reduced to sulphur in most cases. A: Valence electrons of selenium and fluorine are 6 and 7 respectively. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. It is biotic … What is Iodoform? The explanation is on point but using simpler language would make it easier to understand. These 8 electrons are…. How do you find the total number of valence electrons for OF2? Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. It is a tautomer of sulfonic acid. Characteristics and Uses Read More ». A: The electron configuration of Cl is 1s22s22p63s23p5 so, it has 7 valence electrons and that of O is…. A: Octet rule states that an atom should have 8 electrons in its outer shell to complete its octet. The least powerful is sulfuric acid.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial.
Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. A: We have to see the octet of atoms. Get in touch with us. The least powerful is sulfuric acid. By having an odd number of valence electrons. View More Comments. At room temperature or under normal conditions, it reacts with alkyl carbonates. Apart from sulphurous acid uses, there are some toxicity issues. It is an abrasive oxoacid of sulphur and a tautomeric form of sulfonic acid. What is the total number of… A: a The total number of available valence electrons in structure of water is 8.

It is a pity, that now I can not express - it is very occupied. But I will return - I will necessarily write that I think on this question.
You commit an error. I can defend the position. Write to me in PM, we will discuss.