Lewis structure of sf2
Q: How would you prepare Q: For iron in a low spin state O a. A: We have to find the low spin state of iron. For that first we need to write electronic….
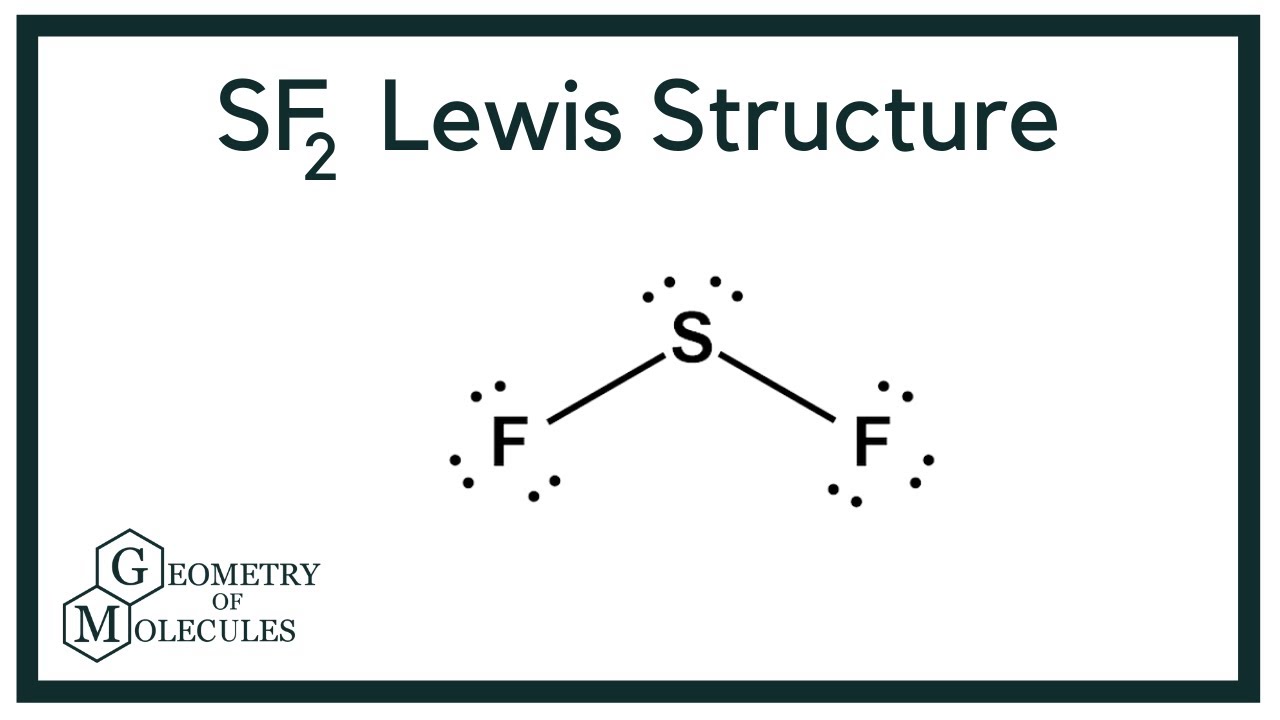
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape. Lewis Structure of SF2, the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. The two lone pairs of electrons push the Fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent. Your physics assignments can be a real challenge, and the due date can be really close — feel free to use our assistance and get the desired result. Be sure that math assignments completed by our experts will be error-free and done according to your instructions specified in the submitted order form.
Lewis structure of sf2
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements.
Valence Electrons of Elements.
Wiki User. The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Ensure that Flourine is not breaking the octet rule as it is a smaller atom and is not known to do so. They should both have neutral charges so no charge is required to be written. So sulfur will end up with 6 electron in its valence level. This molecule doesn't follow the octet rule. No, SF6 doesn't.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. So, Sulphur Difluoride has a total of 20 valence electrons. Lewis Structure is the pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. And now that we know the total valence electrons of SF2, we will start making the Lewis Dot Structure for this molecule.
Lewis structure of sf2
Discover the essentials of the SF2 molecule in our detailed blog post. Learn about the SF2 Lewis Structure, get insights into its molecular geometry, and explore the hybridization process. This guide is ideal for students and chemistry fans looking to expand their knowledge in molecular science, presented in a clear and easy-to-understand format. Lewis structures are a useful tool in chemistry for visualizing the arrangement of atoms and electrons in a molecule. In this guide, we will learn how to draw the Lewis structure of SF2 sulfur difluoride step by step. To determine the total number of valence electrons in SF2, we need to look at the periodic table. Sulfur is in group 16, so it has 6 valence electrons. Fluorine is in group 17, so each fluorine atom has 7 valence electrons.
Caterpillar trainers uk
Lewis Dot Symbols. Note that in such shorthand ring structures, each point where lines meet is a carbon atom and that the hydrogen atoms bonded to the carbon atoms in the rings have been omitted. Diprotic Acids and Bases. However, they need to be checked by the moderator before being published. Naming Amines. F-S-F The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. A: Answer:. Intensive vs. Lewis Structure of SF2, the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. Filtration and Evaporation. Lewis Dot Structures: Ions. Similar questions. Osmotic Pressure. Ester Reactions: Saponification. How many equivalent Lewis structures are necessary to describe the bonding in SF2?
The Sulfur atom S is at the center and it is surrounded by 2 Fluorine atoms F. The Sulfur atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs.
Naming Alkynes. Amphoteric Species. What is the molarity of the solution? Root Mean Square Speed. Crystal Field Theory: Octahedral Complexes. Henry's Law Calculations. Molecular Geometry. KMnO4 : It is strong…. Equilibrium Constant Calculations. Peroxide and Superoxide Reactions. Calculate the price. Mark as completed.

I apologise, but, in my opinion, you are not right. I am assured. Let's discuss it. Write to me in PM, we will talk.