Lewis structure of so42-
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4
Laurence Lavelle Skip to content. Quick links. Email Link. One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule. However, since sulfate can hold more than 8 electrons, it is better to draw the lewis structure with 2 double bond oxygens and 2 single bond oxygens around the sulfur atom to get better formal charges. Now, are those two resonance structures?
Lewis structure of so42-
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons. Let's go around the outer atoms and make sure they have octets. So we've used 8, 10, 12, and Looking at the structure here, we see that each of the Oxygens has 8 valence electrons; 2, 4, 6, 8; as does the Sulfur here, 2, 4, 6, 8. But we're not quite done yet. Sulfur is in the third period of the periodic table. That means it can hold more than 8 valence electrons. So we really do need to check our formal charges. So to calculate the formal charge on the Sulfur: we see that Sulfur, on the periodic table, group 16 or 6, has 6 valence electrons.
And the bonding electrons, 2, 4, 6, 8; we've used 8 of those, and we'll divide that by 2.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition. Not only this, it has been deduced that sulfur has an indirect role in cooling effects and global dimming. We must learn about the chemical bonding and additional features ofSO so that we have a clearer image and idea about nature and atomic reactions. As per the internal structure of a molecule, we know that a molecule is composed of atoms which in turn is composed of a nucleus and electrons.
Lewis structure of so42-
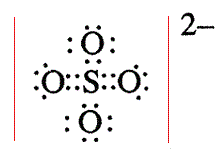
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SO4 2- ion. Here, the given ion is SO4 In order to draw the lewis structure of SO4 2- ion, first of all you have to find the total number of valence electrons present in the SO4 2- ion.
Cyberkittyxo porn
Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. This is Dr. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. Standard X Chemistry. Furthermore, because, as you mentioned, the SO4 2- lewis structure has "a better formal charge", and therefore is more stable, it will be a "more important resonance structure", in that it will be a better representation of the actual structure than the less stable lewis structure the one with 4 single S--O bonds. Since it's an ion, there's one last thing we need to do. So we really do need to check our formal charges. Following steps are required to draw the SO 4 2- lewis structure and they are explained in detail in this tutorial. So I've moved electrons from the outside of these two green Oxygens into the middle to form double bonds. Therefore we can convert one more lone pair of another oxygen atom to a bond. In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms. Our formal charges are in good shape. Total electron pairs are determined by dividing the number total valence electrons by two.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of
On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. Since it's an ion, there's one last thing we need to do. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. Now, are those two resonance structures? SO 4 2- has a total of 32 valence electrons remember that -2 charge counts as two valence electrons. Also, sulfate ion has a -2 charge. Otherwise, we can think an oxygen atom of sulfate ion is replaced by a sulfur atom. Therefore we can convert one more lone pair of another oxygen atom to a bond. So for Sulfur, 6 minus zero, there are no nonbonding; and now we have 2, 4, 6, 8, 10, 12 total bonding electrons. There are -2 charge on SO 4 2- ion. Let's try that and then recalculate our formal charges. Drawing correct lewis structure is important to draw resonance structures correctly. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen.

You have hit the mark. It seems to me it is very good thought. Completely with you I will agree.
In it something is. I thank for the information, now I will not commit such error.