Lewis structure seo2
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around lewis structure seo2 atom until each gets an octet. Count the valence electrons in your trial structure
There are 2 double bonds between the Selenium atom Se and each Oxygen atom O. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se. In order to find the total valence electrons in a SeO2 selenium dioxide molecule , first of all you should know the valence electrons present in selenium atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Selenium is a group 16 element on the periodic table.
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4. Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of
This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. The chemical formula SeO 2 represents the chemical compound Selenium Dioxide.
.
The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water. Selenium dioxide reacts with water to produce selenic acid that initiates corrosion in most of the metals. Besides this, selenium dioxide reaches the soil and water through coal or oil combustion and weathering of rocks. Even though selenium is beneficial for living organisms if consumed in smaller amounts but pertains to toxic effects such as deformed embryos and reproductive failure if exposed to larger quantities. Immediate symptoms of selenium dioxide exposure to human beings include burning sensation, irritation to eyes, nausea, headache, etc. There are various methods of preparing selenium dioxide, but the most preferred method is the dehydration of selenous acid. Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. In a typical structure, the atomic symbols of participating atoms are at the center, and valence electrons are in pairs around them.
Lewis structure seo2
This structure helps us understand the arrangement of atoms and the distribution of electrons in the molecule. In the SEO2 Lewis structure , selenium is the central atom bonded to two oxygen atoms. Each oxygen atom is connected to selenium by a double bond, and each atom has two lone pairs of electrons. This arrangement gives SEO2 a bent molecular geometry. Understanding the SEO2 Lewis structure is important in studying the chemical properties and reactions of selenium dioxide. Lewis structure s are a visual representation of the arrangement of atoms and electrons in a molecule. They provide valuable insights into the bond ing and structure of molecules, helping us understand their properties and behavior. By using Lewis structure s, we can determine the number of valence electrons in a molecule and predict its molecular geometry , bond angles, and polarity.
Lewdchat
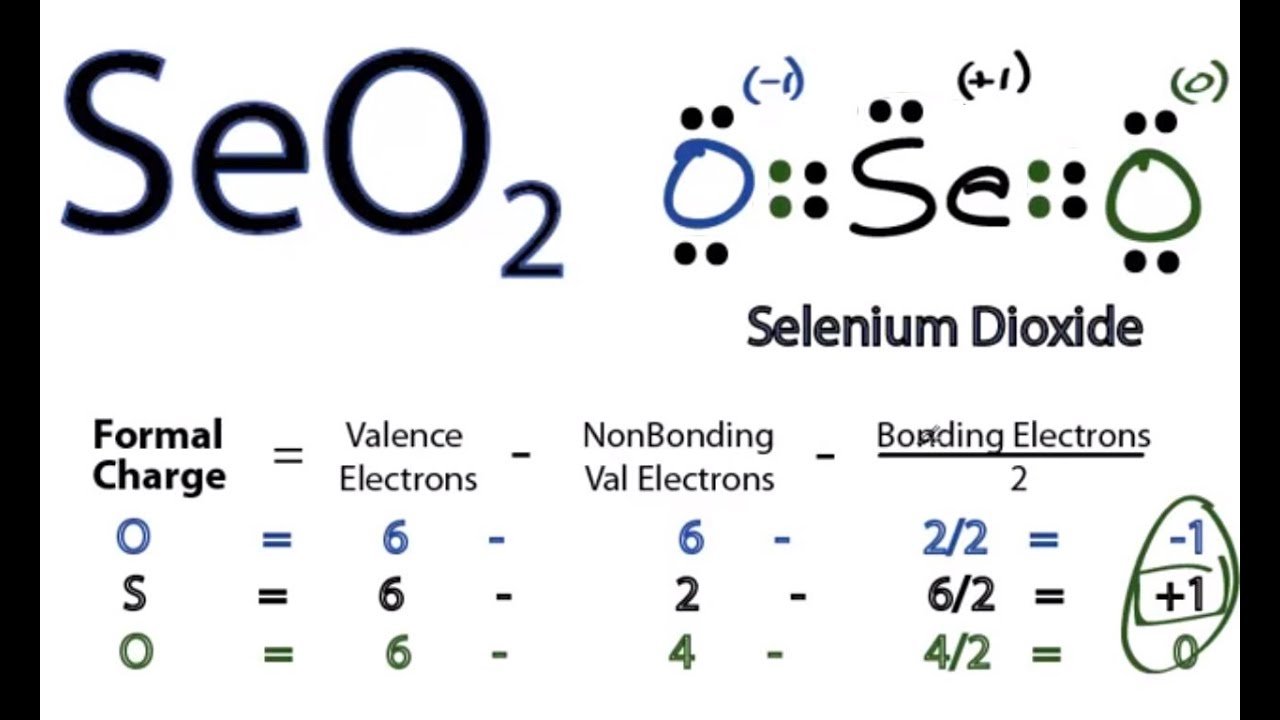
Related questions How is the Lewis structure of an ion written? It is a greenish-yellow crystalline solid with an irritating odor. How is the total number of electrons represented in a Lewis structure determined? Ernest Z. Unfortunately, the selenium atom is not forming an octet here. I write all the blogs after thorough research, analysis and review of the topics. Calculate the formal charge on each atom. In order to find the total valence electrons in a SeO2 selenium dioxide molecule , first of all you should know the valence electrons present in selenium atom as well as oxygen atom. Unfamiliar atomic species interact with one another to form new substances built on chemical bonds. The presence of positive and negative formal charges tells us that this may not be the most stable structure for SeO 2. You have to put these 2 electrons on the central selenium atom in the above sketch of SeO2 molecule. Therefore, the Lewis structure of SeO 2 is given below as:. Now here the given molecule is SeO2 selenium dioxide and it contains selenium atom Se and oxygen atoms O. After shifting this electron pair, the central selenium atom will get 2 more electrons and thus its total electrons will become 8. We can generate a structure with zero formal charges if we move a lone pair from the single-bonded "O" to make a double bond to the "S".
Transcript: This is Dr.
In short, now you have to find the formal charge on selenium Se atom as well as oxygen O atoms present in the SeO2 molecule. Impact of this question views around the world. If we compare the electronegativity values of selenium Se and oxygen O then the selenium atom is less electronegative. In this case, that would be Oxygen. That will normally be the least electronegative atom "Se". You can see the number of bonding electrons and nonbonding electrons for each atom of SeO2 molecule in the image given below. In order to check the stability of the central selenium Se atom, we have to check whether it is forming an octet or not. The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. The electron geometry around "Se" is trigonal planar. Selenium is a group 16 element on the periodic table. It is determined such that the elemental charge on each atom is closest to zero. After shifting this electron pair, the central selenium atom will get 2 more electrons and thus its total electrons will become 8.

Willingly I accept. The question is interesting, I too will take part in discussion. Together we can come to a right answer. I am assured.
Your inquiry I answer - not a problem.