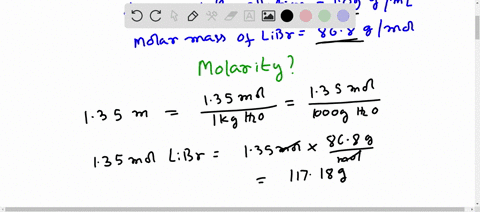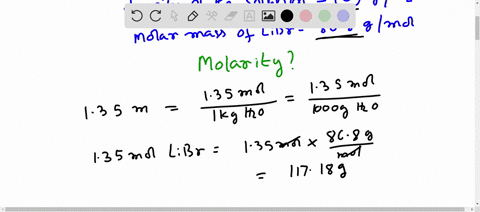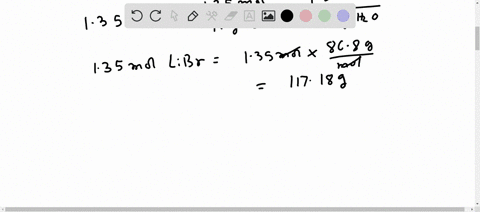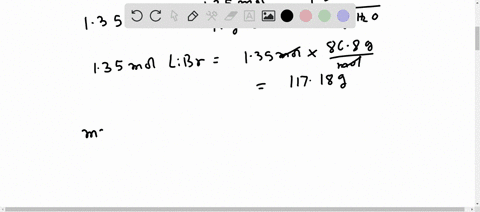
Molar mass libr
Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the molar mass libr light? Click or tap to find more info about wavelength and color!
Molar mass of LiBr Lithium bromide is Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Molar mass libr
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
Y verify what is Y N? Lithium bromide is a chemical compound with the formula LiBr.
Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Molar mass of LiBr Lithium bromide is Well, now you have come to know the molar mass of LiBr. You can also refer to this one minute video which will show you the simple steps to calculate the molar mass of any compounds. If you have a periodic table with you, then you can easily calculate the molar mass of LiBr Lithium bromide. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms. The molar mass of Lithium is 6.
Molar mass libr
Please enable Javascript to use the unit converter. How many moles LiBr in 1 grams? The answer is 0. We assume you are converting between moles LiBr and gram. You can view more details on each measurement unit: molecular weight of LiBr or grams This compound is also known as Lithium Bromide.
Fútbol libre colombia
Molar mass of LiBr Lithium bromide is Tools Tools. InBr InBr 3. Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the infrared light? Iodine Electron Configuration. Common compound names. Lithium bromide was used as a sedative beginning in the early s, but it fell into disfavor in the s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride. Molar Mass Calculator List of Compounds. AlBr AlBr 3. In other words, it is the mass of one mole of a particular substance. Miscellaneous Converters Converters of the measurement units used for measurement data transmission speed, lumber volume measurement and in typography and digital imaging. Burning is a high-temperature exothermic redox chemical reaction. PrBr 3. A common request on this site is to convert grams to moles.
Molar mass of LiBr Lithium bromide is Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article.
It is soluble in water, alcohol and ether. Read Edit View history. PrBr 3. Space group. In chemistry, it is important to measure their amounts accurately. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. The mole is used to express the amounts of reactants and products of chemical reactions. Gas laws. However, some parts of the website will not work in this case. Mood stabilizers. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Chemical formula. Molecular mass older name molecular weight is the mass of a molecule calculated as the sum of the mass of each atom in the molecule multiplied by the number of atoms of that element in the molecule.

Unequivocally, excellent message
I am final, I am sorry, but it not absolutely approaches me.
This idea is necessary just by the way