Molar mass of sodium hydroxide
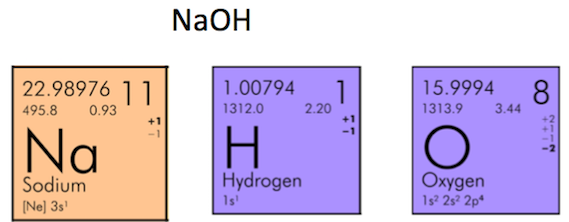
Molar mass of NaOH Sodium hydroxide is Then, lookup atomic weights for each element in periodic table : Na:
Sodium hydroxide , also known as lye and caustic soda , [1] [2] is an inorganic compound with the formula NaOH. Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures and may cause severe chemical burns. It is highly soluble in water , and readily absorbs moisture and carbon dioxide from the air. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
Molar mass of sodium hydroxide
.
Sodium hydroxide was first prepared by soap makers. European soap makers also followed this recipe. The pressurization is due to the hydrogen gas which is produced in the reaction between sodium hydroxide and aluminium:.
.
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles. You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. To understand the topic as a whole, you will want to learn the mole definition, read a paragraph about the molarity units, as well as read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, we prepared for you some interesting examples of molar solutions and a short step-by-step tutorial on how to calculate the molarity of a concentrated solution. At the end, you can learn the titration definition and discover how to find the molar concentration using the titration process! The molarity calculator is straightforward and convenient, and you will find that out soon.
Molar mass of sodium hydroxide
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Porn lesbiche
Ba OH 2. However, solutions of NaOH can be easily supercooled by many degrees, which allows the formation of hydrates including the metastable ones from solutions with different concentrations. Sodium hydroxide is used in the manufacture of sodium salts and detergents, pH regulation, and organic synthesis. The alkali dissolves greases to produce water soluble products. Zr OH 4. Retrieved January 15, OCLC Chemical compound. Sodium hydroxide has been used for detection of carbon monoxide poisoning , with blood samples of such patients turning to a vermilion color upon the addition of a few drops of sodium hydroxide. Worldwide production in was approximately 60 million tons, while demand was 51 million tons. Despite solubility in propylene glycol it is unlikely to replace water in saponification due to propylene glycol's primary reaction with fat before reaction between sodium hydroxide and fat. CAS Number. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. CO 2 has one carbon atom and two oxygen atoms.
You can go from moles of sodium hydroxide, "NaOH" , to grams of sodium hydroxide by using a conversion factor called molar mass. The molar mass tells you the mass of exactly one mole of a given substance.
Dy OH 3. Sodium hydroxide is used in some relaxers to straighten hair. Read Edit View history. It can dissolve grease , oils , fats and protein -based deposits. Ho OH 3. Sodium hydroxide is used with hydrochloric acid to balance pH. Ac OH 3. August 29, Organic and Medicinal Chemistry Letters. Sodium hydroxide is frequently used as an industrial cleaning agent where it is often called "caustic". Sodium hydroxide is sometimes used during water purification to raise the pH of water supplies. The following colours are observed:. Ga OH 3. Acidity p K a.

I consider, that you commit an error. I can prove it. Write to me in PM, we will talk.
Unsuccessful idea
Certainly. It was and with me.