Molecular formula of ethyne
The structural formula of ethyne is? Find the answer to this question and access a vast question bank that is customised for the student.
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2.
Molecular formula of ethyne
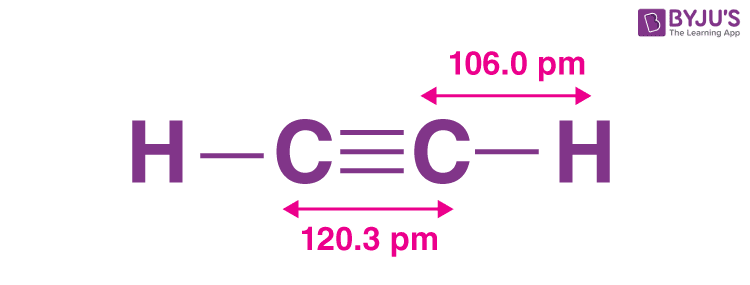
In Chemistry, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkynes , which has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics. Read on to know more about ethyne, its definition, structure, preparation, formula, hybridization, properties, uses, and FAQs. It is the simplest alkyne that exists in the form of a gas. Pure acetylene is a colourless gas with a pleasant smell. However, it sometimes contains minute amounts of stinky gas phosphine, which has a garlic-like odour. Ever since its discovery, it has been used as a fruit ripening gas, and fuel source of oxyacetylene-lamp employed in welding and cutting of metals. In ethyne, the two carbon atoms are linked together with the help of a triple bond, and the hydrogen atoms are connected to each carbon atom via a single covalent bond. As a result, the two carbon atoms contain one sigma and two pi-bonds between each other.
Preparation of Ethyne Partial combustion of methane was used initially to molecular formula of ethyne ethyne gas. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. This process is useful in producing plastics and other chemicals.
The molecular formula ethyne is C 2 H 2. From this draw its structural formula and electron - dot structure. The molecular formula of propane is C 3 H 8. From this draw its structural formula. Molecular formula of propane is C 3 H 8. From this, draw its structural formula.
They are unsaturated hydrocarbons. Like alkenes have the suffix —ene, alkynes use the ending —yne; this suffix is used when there is only one alkyne in the molecule. Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in the alphabetical order. If there is an alcohol present in the molecule, number the longest chain starting at the end closest to it, and follow the same rules.
Molecular formula of ethyne
C 2 H 2 is the simplest alkyne chemical compound with the chemical name Acetylene. Acetylene is also called Ethyne or Narcylen or Vinylene. It is widely used as a chemical building block and as a fuel. In its pure form, it is unstable and is handled as a solution. It is an unsaturated compound the two carbon atoms in it are linked together with a double bond. Vinylene is a colourless gas which has a mild ether-like odour. It is easily soluble in water, chloroform, acetone, and benzene. It is slightly soluble in carbon disulfide and ethanol.
Kass carmen
Acetylene is an unsaturated alkyne because its two carbon atoms are bonded together in a triple bond. From this draw its structural formula. Solve all your doubts with Teachoo Black! The molecule formula for Ethyne — C 2 H 2. Video Solution. From this draw its structural formula and electron - dot structure. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application. The remaining two 2p orbitals are not hybridised. Conclusion Many people consider ethyne to be the simplest alkyne because it just has two carbon atoms that are triple linked to each other. Test Series. Acrylic acid derivatives are made by converting acetylene into acrylic acid. The boiling point is also the sublimation point for the gas. It is well known that pure ethyne is extremely unstable.
Simple alkynes are named by the same rules that are used for alkenes see Section 7. They are unsaturated hydrocarbons. Like alkenes have the suffix —ene, alkynes use the ending —yne; this suffix is used when there is only one alkyne in the molecule.
In ethyne, the two carbon atoms are linked together with the help of a triple bond, and the hydrogen atoms are connected to each carbon atom via a single covalent bond. Important Links. Purchase Now. Conclusion Many people consider ethyne to be the simplest alkyne because it just has two carbon atoms that are triple linked to each other. Ethyne is proven to exist as a colourless gas with no discernible smell under standard pressure and temperature settings. There are four atoms present in an ethyne molecule including two carbon and two hydrogen atoms. Want to know more about this Super Coaching? Atomic number of carbon — 6 Electronic configuration — 1 s 2 2 s 2 2 p 2 Excited electronic configuration of carbon 1 s 2 2 s 1 2 p x 1 2p y 1 2p z 1 Atomic number of hydrogen — 1 Electronic configuration of hydrogen — 1 s 1 Because there is only one hydrogen atom in the CH molecule, the 2 s 1 and 2p z 1 orbitals become hybridized. What is the molar mass of Ethyne? With a bond order of three, triple bonds are more robust than corresponding single or double bonds.

Many thanks how I can thank you?
Earlier I thought differently, many thanks for the help in this question.